DC-based vaccine loaded with acid-eluted peptides in acute myeloid leukemia: the importance of choosing the best elution method
- PMID: 16676183
- PMCID: PMC11030744
- DOI: 10.1007/s00262-006-0170-6
DC-based vaccine loaded with acid-eluted peptides in acute myeloid leukemia: the importance of choosing the best elution method
Abstract
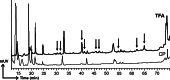
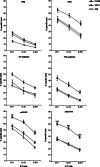
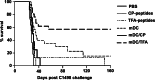
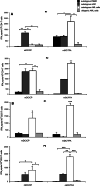
Tumor-associated peptides isolated by acid elution are frequently used for therapeutic immunization against various tumors both in mice and in humans. In acute myeloid leukemia (AML), the frequent accessibility of a large tumor burden allows for extraction of peptides from leukemia cells by using either citrate-phosphate (CP) or trifluoroacetic acid (TFA) buffer. To develop an optimal immunotherapeutic protocol for AML patients, we evaluated both in mice and in humans, the immunogenicity of peptides eluted from leukemia cells with the two acids (TFA or CP). Although ex vivo studies in mice showed that both prophylactic immunizations with mature dendritic cells (DC) loaded with TFA-peptides (DC/TFA), or CP-peptides (DC/CP), were able to stimulate specific antileukemia immune responses, only vaccination with DC/TFA was able to prevent leukemia outgrowth. Moreover, in humans, only DC/TFA generated significant antileukemia CD4(+) and cytotoxic CD8(+) T cell responses in vitro. In summary, these data demonstrate that the choice of the acid elution procedure to isolate immunogenic peptides strongly influences the efficacy of the antileukemia immune responses. These finding raise essential considerations for the development of immunotherapeutic protocols for cancer patients. In our model, our results argue for the use of the TFA elution method to extract immunogenic AML-associated peptides.
Figures
Similar articles
-
Autologous peptides eluted from acute myeloid leukemia cells can be used to generate specific antileukemic CD4 helper and CD8 cytotoxic T lymphocyte responses in vitro.Haematologica. 2005 Aug;90(8):1050-62. Haematologica. 2005. PMID: 16079104
-
Dramatic efficacy improvement of a DC-based vaccine against AML by CD25 T cell depletion allowing the induction of a long-lasting T cell response.Cancer Immunol Immunother. 2009 Oct;58(10):1669-77. doi: 10.1007/s00262-009-0678-7. Epub 2009 Feb 19. Cancer Immunol Immunother. 2009. PMID: 19225777 Free PMC article.
-
Eliciting cytotoxic T lymphocytes against acute myeloid leukemia-derived antigens: evaluation of dendritic cell-leukemia cell hybrids and other antigen-loading strategies for dendritic cell-based vaccination.Cancer Immunol Immunother. 2002 Aug;51(6):299-310. doi: 10.1007/s00262-002-0284-4. Epub 2002 Jun 14. Cancer Immunol Immunother. 2002. PMID: 12111118 Free PMC article.
-
Employing the immunological synapse in AML: development of leukemic dendritic cells for active specific immunization.Immunobiology. 2005;210(2-4):249-57. doi: 10.1016/j.imbio.2005.05.019. Immunobiology. 2005. PMID: 16164032 Review.
-
New generation dendritic cell vaccine for immunotherapy of acute myeloid leukemia.Cancer Immunol Immunother. 2014 Oct;63(10):1093-103. doi: 10.1007/s00262-014-1600-5. Epub 2014 Sep 4. Cancer Immunol Immunother. 2014. PMID: 25186611 Free PMC article. Review.
Cited by
-
Dosage and organic acid residue of myelin oligodendrocyte glycoprotein35-55 peptide influences immunopathology and development of Bacillus Calmette-Guérin induced experimental autoimmune encephalomyelitis.Exp Anim. 2025 Jan 10;74(1):16-30. doi: 10.1538/expanim.24-0012. Epub 2024 Jul 10. Exp Anim. 2025. PMID: 38987201 Free PMC article.
-
Cytotoxic T-cells as imaging probes for detecting glioma.World J Clin Oncol. 2010 Nov 10;1(1):3-11. doi: 10.5306/wjco.v1.i1.3. World J Clin Oncol. 2010. PMID: 21603304 Free PMC article.
-
Activated T cell therapy targeting glioblastoma cancer stem cells.Sci Rep. 2023 Jan 5;13(1):196. doi: 10.1038/s41598-022-27184-w. Sci Rep. 2023. PMID: 36604465 Free PMC article.
-
Therapeutic targeting of naturally presented myeloperoxidase-derived HLA peptide ligands on myeloid leukemia cells by TCR-transgenic T cells.Leukemia. 2014 Dec;28(12):2355-66. doi: 10.1038/leu.2014.131. Epub 2014 Apr 16. Leukemia. 2014. PMID: 24736212
-
Current approaches in dendritic cell generation and future implications for cancer immunotherapy.Cancer Immunol Immunother. 2007 Oct;56(10):1513-37. doi: 10.1007/s00262-007-0334-z. Epub 2007 May 15. Cancer Immunol Immunother. 2007. PMID: 17503040 Free PMC article. Review.
References
-
- Avigan D, Vasir B, Gong J, Borges V, Wu Z, Uhl L, Atkins M, Mier J, McDermott D, Smith T, Giallambardo N, Stone C, Schadt K, Dolgoff J, Tetreault JC, Villarroel M, Kufe D. Fusion cell vaccination of patients with metastatic breast and renal cancer induces immunological and clinical responses. Clin Cancer Res. 2004;10:4699–4708. doi: 10.1158/1078-0432.CCR-04-0347. - DOI - PubMed
-
- Bellone M, Iezzi G, Manfredi AA, Protti MP, Dellabona P, Casorati G, Rugarli C. In vitro priming of cytotoxic T lymphocytes against poorly immunogenic epitopes by engineered antigen-presenting cells. Eur J Immunol. 1994;24:2691–2698. - PubMed
-
- Boyer MW, Orchard PJ, Gorden KB, Anderson PM, McLvor RS, Blazar BR. Dependency on intercellular adhesion molecule recognition and local interleukin-2 provision in generation of an in vivo CD8+ T-cell immune response to murine myeloid leukemia. Blood. 1995;85:2498–2506. - PubMed
-
- Bradner WT, Pindell MH. Myeloid leukemia C-1498 as a screen for cancer chemotherapeutic agents. Cancer Res. 1966;26:375–390. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

