Frataxin, iron-sulfur clusters, heme, ROS, and aging
- PMID: 16677095
- PMCID: PMC1805116
- DOI: 10.1089/ars.2006.8.506
Frataxin, iron-sulfur clusters, heme, ROS, and aging
Abstract
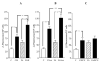
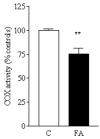
A deficiency in mitochondrial frataxin causes an increased generation of mitochondrial reactive oxygen species (ROS), which may contribute to the cell degenerative features of Friedreich's ataxia. In this work the authors demonstrate mitochondrial iron-sulfur cluster (ISC) defects and mitochondrial heme defects, and suggest how both may contribute to increased mitochondrial ROS in lymphoblasts from human patients. Mutant cells are deficient in the ISC-requiring mitochondrial enzymes aconitase and succinate dehydrogenase, but not in the non-ISC mitochondrial enzyme citrate synthase; also, the mitochondrial iron-sulfur scaffold protein IscU2 co-immunoprecipitates with frataxin in vivo. Presumably as a consequence of the iron-sulfur cluster defect, cytochrome c heme is deficient in mutants, as well as heme-dependent Complex IV. Mitochondrial superoxide is elevated in mutants, which may be a consequence of cytochrome c deficiency. Hydrogen peroxide, glutathione peroxidase activity, and oxidized glutathione (GSSG) are each elevated in mutants, consistent with activation of the glutathione peroxidase pathway. Mutant status blunted the effects of Complex III and IV inhibitors, but not a Complex I inhibitor, on superoxide production. This suggests that heme defects late in the electron transport chain of mutants are responsible for increased mutant superoxide. The impact of ISC and heme defects on ROS production with age are discussed.
Figures

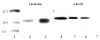


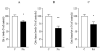
References
-
- Atamna H, Liu J, Ames BN. Heme deficiency selectively interrupts assembly of mitochondrial complex IV in human fibroblasts: revelance to aging. J Biol Chem. 2001;276:48410–48416. - PubMed
-
- Bidichandani SI, Purandare SM, Taylor EE, Gumin G, Machkhas H, Harati Y, Gibbs RA, Ashizawa T, Patel PI. Somatic sequence variation at the Friedreich ataxia locus includes complete contraction of the expanded GAA triplet repeat, significant length variation in serially passaged lymphoblasts and enhanced mutagenesis in the flanking sequence. Hum Mol Genet. 1999;8:2425–2436. - PubMed
-
- Birch-Machin MA, Turnbull DM. Assaying mitochondrial respiratory complex activity in mitochondria isolated from human cells and tissues. Methods Cell Biol. 2001;65:97–117. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

