Genetic effects of oxidative DNA damages: comparative mutagenesis of the imidazole ring-opened formamidopyrimidines (Fapy lesions) and 8-oxo-purines in simian kidney cells
- PMID: 16679449
- PMCID: PMC1458282
- DOI: 10.1093/nar/gkl099
Genetic effects of oxidative DNA damages: comparative mutagenesis of the imidazole ring-opened formamidopyrimidines (Fapy lesions) and 8-oxo-purines in simian kidney cells
Erratum in
- Nucleic Acids Res. 2006;34(9):2845
Abstract
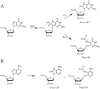
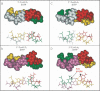
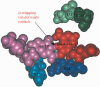
Fapy.dG and 8-oxo-7,8-dihydro-2'-deoxyguanosine (8-oxo-dG) are formed in DNA by hydroxyl radical damage. In order to study replication past these lesions in cells, we constructed a single-stranded shuttle vector containing the lesion in 5'-TGT and 5'-TGA sequence contexts. Replication of the modified vector in simian kidney (COS-7) cells showed that Fapy.dG is mutagenic inducing primarily targeted Fapy.G-->T transversions. In the 5'-TGT sequence mutational frequency of Fapy.dG was approximately 30%, whereas in the 5'-TGA sequence it was approximately 8%. In parallel studies 8-oxo-dG was found to be slightly less mutagenic than Fapy.dG, though it also exhibited a similar context effect: 4-fold G-->T transversions (24% versus 6%) occurred in the 5'-TGT sequence relative to 5'-TGA. To investigate a possible structural basis for the higher G-->T mutations induced by both lesions when their 3' neighbor was T, we carried out a molecular modeling investigation in the active site of DNA polymerase beta, which is known to incorporate both dCTP (no mutation) and dATP (G-->T substitution) opposite 8-oxo-G. In pol beta, the syn-8-oxo-G:dATP pair showed greater stacking with the 3'-T:A base pair in the 5'-TGT sequence compared with the 3'-A:T in the 5'-TGA sequence, whereas stacking for the anti-8-oxo-G:dCTP pair was similar in both 5'-TGT and 5'-TGA sequences. Similarly, syn-Fapy.G:dATP pairing showed greater stacking in the 5'-TGT sequence compared with the 5'-TGA sequence, while stacking for anti-Fapy.G:dCTP pairs was similar in the two sequences. Thus, for both lesions less efficient base stacking between the lesion:dATP pair and the 3'-A:T base pair in the 5'-TGA sequence might cause lower G-->T mutational frequencies in the 5'-TGA sequence compared to 5'-TGT. The corresponding lesions derived from 2'-deoxyadenosine, Fapy.dA and 8-oxo-dA, were not detectably mutagenic in the 5'-TAT sequence, and were only weakly mutagenic (<1%) in the 5'-TAA sequence context, where both lesions induced targeted A-->C transversions. To our knowledge this is the first investigation using extrachromosomal probes containing a Fapy.dG or Fapy.dA site-specifically incorporated, which showed unequivocally that in simian kidney cells Fapy.G-->T substitutions occur at a higher frequency than 8-oxo-G-->T and that Fapy.dA is very weakly mutagenic, as is 8-oxo-dA.
Figures



Similar articles
-
Studies on the replication of the ring opened formamidopyrimidine, Fapy.dG in Escherichia coli.Biochemistry. 2007 Sep 4;46(35):10202-12. doi: 10.1021/bi700628c. Epub 2007 Aug 11. Biochemistry. 2007. PMID: 17691820
-
Synergistic effects on mutagenicity of tandem lesions containing 8-oxo-7,8-dihydro-2'-deoxyguanosine or Fapy•dG flanked by a 3' 5-formyl-2'-deoxyuridine in human cells.DNA Repair (Amst). 2023 Sep;129:103527. doi: 10.1016/j.dnarep.2023.103527. Epub 2023 Jun 24. DNA Repair (Amst). 2023. PMID: 37467631 Free PMC article.
-
Structural Dynamics of a Common Mutagenic Oxidative DNA Lesion in Duplex DNA and during DNA Replication.J Am Chem Soc. 2022 May 11;144(18):8054-8065. doi: 10.1021/jacs.2c00193. Epub 2022 May 2. J Am Chem Soc. 2022. PMID: 35499923 Free PMC article.
-
The formamidopyrimidines: purine lesions formed in competition with 8-oxopurines from oxidative stress.Acc Chem Res. 2012 Apr 17;45(4):588-97. doi: 10.1021/ar2002182. Epub 2011 Nov 11. Acc Chem Res. 2012. PMID: 22077696 Free PMC article. Review.
-
The Influence of 2'-Deoxyguanosine Lesions on the Electronic Properties of OXOG:::C Base Pairs in Ds-DNA: A Comparative Analysis of Theoretical Studies.Molecules. 2024 Aug 8;29(16):3756. doi: 10.3390/molecules29163756. Molecules. 2024. PMID: 39202837 Free PMC article. Review.
Cited by
-
Replication of the 2,6-diamino-4-hydroxy-N(5)-(methyl)-formamidopyrimidine (MeFapy-dGuo) adduct by eukaryotic DNA polymerases.Chem Res Toxicol. 2012 Aug 20;25(8):1652-61. doi: 10.1021/tx300113e. Epub 2012 Jul 6. Chem Res Toxicol. 2012. PMID: 22721435 Free PMC article.
-
Unexpected non-Hoogsteen-based mutagenicity mechanism of FaPy-DNA lesions.Nat Chem Biol. 2013 Jul;9(7):455-61. doi: 10.1038/nchembio.1254. Epub 2013 May 19. Nat Chem Biol. 2013. PMID: 23685671
-
Mutagenic potential of DNA-peptide crosslinks mediated by acrolein-derived DNA adducts.Mutat Res. 2008 Jan 1;637(1-2):161-72. doi: 10.1016/j.mrfmmm.2007.08.001. Epub 2007 Aug 7. Mutat Res. 2008. PMID: 17868748 Free PMC article.
-
An evolutionary footprint of age-related natural selection in mitochondrial DNA.J Mol Evol. 2008 Oct;67(4):412-7. doi: 10.1007/s00239-008-9163-8. Epub 2008 Sep 23. J Mol Evol. 2008. PMID: 18810522
-
Inhibition by 4-(4-Bromo-2-oxo-3H-benzimidazol-1-yl)-N-(4-iodophenyl)piperidine-1-carboxamide (TH5487) of the Activity of Human 8-Oxoguanine DNA Glycosylase-1 (OGG1) for the Excision of 2,6-Diamino-4-hydroxy-5-formamidopyrimidine, 4,6-Diamino-5-formamidopyrimidine, and 8-Oxoguanine from Oxidatively Damaged DNA.Biochemistry. 2025 Apr 15;64(8):1788-1796. doi: 10.1021/acs.biochem.4c00419. Epub 2025 Apr 3. Biochemistry. 2025. PMID: 40179276 Free PMC article.
References
-
- Beckman K.B., Ames B.N. Oxidative decay of DNA. J. Biol. Chem. 1997;272:19633–19636. - PubMed
-
- Anson R.M., Sentürker S., Dizdaroglu M., Bohr V.A. Measurement of oxidatively induced base lesions in liver nuclear and mitochodrial DNA from Wistar rats of different ages. Free Radic. Biol. Med. 1999;27:456–462. - PubMed
-
- Cooke M.S., Evans M.D., Dizdaroglu M., Lunec J. Oxidative DNA damage: mechanisms, mutation, and disease. FASEB J. 2003;17:1195–1214. - PubMed
-
- Téoule R. Radiation induced DNA damage and its repair. Int. J. Radiat. Biol. 1987;51:573–589. - PubMed
-
- Breen A.P., Murphy J.A. Reactions of oxyl radical with DNA. Free Radic. Biol. Med. 1995;18:1033–1077. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- CA74954/CA/NCI NIH HHS/United States
- R56 CA076163/CA/NCI NIH HHS/United States
- CA50432/CA/NCI NIH HHS/United States
- R56 ES009127/ES/NIEHS NIH HHS/United States
- ES03775/ES/NIEHS NIH HHS/United States
- ES09127/ES/NIEHS NIH HHS/United States
- R01 CA050432/CA/NCI NIH HHS/United States
- R01 CA074954/CA/NCI NIH HHS/United States
- R01 ES009127/ES/NIEHS NIH HHS/United States
- R01 ES003775/ES/NIEHS NIH HHS/United States
- R01 CA076163/CA/NCI NIH HHS/United States
- CA47995/CA/NCI NIH HHS/United States
- R01 ES013324/ES/NIEHS NIH HHS/United States
- ES013324/ES/NIEHS NIH HHS/United States
- P01 CA047995/CA/NCI NIH HHS/United States
- CA76163/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

