The Galpha protein controls a pH-dependent signal path to the induction of phytoalexin biosynthesis in Eschscholzia californica
- PMID: 16679461
- PMCID: PMC1475490
- DOI: 10.1105/tpc.105.035121
The Galpha protein controls a pH-dependent signal path to the induction of phytoalexin biosynthesis in Eschscholzia californica
Abstract
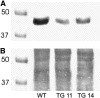
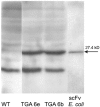
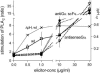
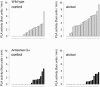
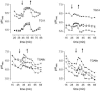
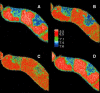
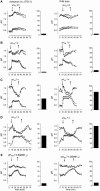
The function of a Galpha protein in the elicitation of phytoalexin (benzophenanthridine) biosynthesis was characterized in cultured cells of California poppy (Eschscholzia californica). Both the decrease of Galpha content via antisense transformation and the expression of recombinant anti-Galpha single-chain antibodies strongly impaired the induction of alkaloid biosynthesis by low elicitor concentrations. All transgenic cell types were deficient in two elicitor-triggered early signal events: activation of phospholipase A2 (PLA2) and efflux of vacuolar protons. The lacking H+ efflux could be restored (1) by adding lysophosphatidylcholine (LPC), a product of PLA2 activity, to vacuoles in situ and (2) by exposing intact cells to isotonic, near-neutral HEPES buffers. The latter treatment induced alkaloid biosynthesis in the absence of elicitor and in Galpha-deficient cells. We conclude that Galpha mediates the stimulation of PLA2 by low elicitor concentrations and that the resulting peak of LPC initiates a transient efflux of vacuolar protons. In this way, an acidic peak of the cytoplasmic pH is generated that causes the expression of enzymes of phytoalexin production independent of the hypersensitive response.
Figures





References
-
- Aharon, G.S., Gelli, A., Snedden, W.A., and Blumwald, E. (1998). Activation of a plant plasma membrane Ca2+ channel by TG α1, a heterotrimeric G protein alpha-subunit homologue. FEBS Lett. 424 17–21. - PubMed
-
- Armero, J., and Tena, M. (2001). Possible role of plasma membrane H+-ATPase in the elicitation of phytoalexin and related isoflavone root secretion in chickpea (Cicer arietinum L.) seedlings. Plant Sci. 161 791–798.
-
- Artsaenko, O., Peisker, M., zur Nieden, U., Fiedler, U., Weiler, E.W., Müntz, K., and Conrad, U. (1995). Expression of a single chain Fv antibody against abscisic acid creates a wilty phenotype in transgenic tobacco. Plant J. 8 745–750. - PubMed
-
- Artsaenko, O., Philipps, J., Fiedler, U., and Peisker, M., and Conrad, U. (1999). Intracellular immunomodulation in plants: A new tool for the investigation of phytohormones. In Recombinant Antibodies: Applications in Plant Science and Plant Pathology, K. Harper and A. Ziegler, eds (London: Taylor & Francis), pp. 145–156.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

