Exon ligation is proofread by the DExD/H-box ATPase Prp22p
- PMID: 16680161
- PMCID: PMC3729281
- DOI: 10.1038/nsmb1093
Exon ligation is proofread by the DExD/H-box ATPase Prp22p
Abstract
To produce messenger RNA, the spliceosome excises introns from precursor (pre)-mRNA and splices the flanking exons. To establish fidelity, the spliceosome discriminates against aberrant introns, but current understanding of such fidelity mechanisms is limited. Here we show that an ATP-dependent activity represses formation of mRNA from aberrant intermediates having mutations in any of the intronic consensus sequences. This proofreading activity is disabled by mutations that impair the ATPase or RNA unwindase activity of Prp22p, a conserved spliceosomal DExD/H-box ATPase. Further, cold-sensitive prp22 mutants permit aberrant mRNA formation from a mutated 3' splice-site intermediate in vivo. We conclude that Prp22p generally represses splicing of aberrant intermediates, in addition to its known ATP-dependent role in promoting release of genuine mRNA. This dual function for Prp22p validates a general model in which fidelity can be enhanced by a DExD/H-box ATPase.
Figures

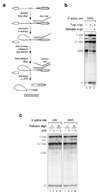
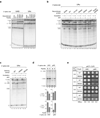

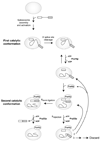
Comment in
-
Splicing fidelity revisited.Nat Struct Mol Biol. 2006 Jun;13(6):472-4. doi: 10.1038/nsmb0606-472. Nat Struct Mol Biol. 2006. PMID: 16757943 No abstract available.
References
-
- Will CL, Lührmann R. Spliceosome structure and function. In: Gesteland RF, Cech TR, Atkins JF, editors. The RNA World. 3rd Edition. New York: Cold Spring Harbor Laboratory Press, New York; 2006. pp. 369–400.
-
- Valadkhan S. snRNAs as the catalysts of pre-mRNA splicing. Curr. Opin Chem. Biol. 2005;9:603–608. - PubMed
-
- Staley JP, Guthrie C. Mechanical devices of the spliceosome: motors, clocks, springs, and things. Cell. 1998;92:315–326. - PubMed
-
- Cordin O, Banroques J, Tanner NK, Linder P. The DEAD-box protein family of RNA helicases. Gene. 2005;367:17–37. - PubMed
-
- Burgess SM, Guthrie C. A mechanism to enhance mRNA splicing fidelity: the RNA-dependent ATPase Prp16 governs usage of a discard pathway for aberrant lariat intermediates. Cell. 1993;73:1377–1391. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

