Neonatal deafness results in degraded topographic specificity of auditory nerve projections to the cochlear nucleus in cats
- PMID: 16680765
- PMCID: PMC2430266
- DOI: 10.1002/cne.20968
Neonatal deafness results in degraded topographic specificity of auditory nerve projections to the cochlear nucleus in cats
Abstract
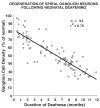
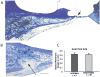
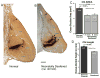
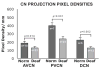
We previously examined the early postnatal maturation of the primary afferent auditory nerve projections from the cat cochlear spiral ganglion (SG) to the cochlear nucleus (CN). In normal kittens these projections exhibit clear cochleotopic organization before birth, but quantitative data showed that their topographic specificity is less precise in perinatal kittens than in adults. Normalized for CN size, projections to the anteroventral (AVCN), posteroventral (PVCN), and dorsal (DCN) subdivisions are all significantly broader in neonates than in adults. By 6-7 postnatal days, projections are proportionate to those of adults, suggesting that significant refinement occurs during the early postnatal period. The present study examined SG projections to the CN in adult cats deafened as neonates by ototoxic drug administration. The fundamental organization of the SG-to-CN projections into frequency band laminae is clearly evident despite severe auditory deprivation from birth. However, when normalized for the smaller CN size in deafened animals, projections are disproportionately broader than in controls; AVCN, PVCN, and DCN projections are 39, 26, and 48% broader, respectively, than predicted if they were precisely proportionate to projections in normal hearing animals. These findings suggest that normal auditory experience and neural activity are essential for the early postnatal development (or subsequent maintenance) of the topographic precision of SG-to-CN projections. After early deafness, the basic cochleotopic organization of the CN is established and maintained into adulthood, but the CN is severely reduced in size and the topographic specificity of primary afferent projections that underlies frequency resolution in the normal central auditory system is significantly degraded.
Copyright 2006 Wiley-Liss, Inc.
Figures
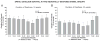



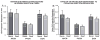
References
-
- Bonham BH, Cheung SW, Benoit G, Schreiner CE. Spatial organization of frequency response areas and rate/level functions in the developing AI. J Neurophysiol. 2004;91:841–854. - PubMed
-
- Brawer JR, Morest DK, Kane EI. The neuronal architecture of the cochlear nucleus of the cat. J Comp Neurol. 1974;155:251–300. - PubMed
-
- Brugge JF, Reale RA, Wilson GF. Sensitivity of auditory cortical neurons of kittens to monaural and binaural high frequency sound. Hearing Res. 1988;34:127–140. - PubMed
-
- Cant NB, Morest DK. The structural basis for stimulus coding in the cochlear nucleus of the cat. In: Berlin C, editor. Hearing Science: Recent Advances. San Diego: College-Hill; 1984. pp. 371–421.
-
- Chalupa LM, Snider CJ. Topographic specificity in the retinocollicular projection of the developing ferret: an anterograde tracing study. J Comp Neurol. 1998;392:35–47. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

