Participation of the tRNA A76 hydroxyl groups throughout translation
- PMID: 16681365
- PMCID: PMC2522371
- DOI: 10.1021/bi060183n
Participation of the tRNA A76 hydroxyl groups throughout translation
Abstract
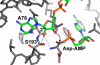
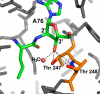
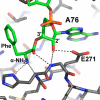
The free 2'-3' cis-diol at the 3'-terminus of tRNA provides a unique juxtaposition of functional groups that play critical roles during protein synthesis. The translation process involves universally conserved chemistry at almost every stage of this multistep procedure, and the 2'- and 3'-OHs are in the immediate vicinity of chemistry at each step. The cis-diol contribution affects steps ranging from tRNA aminoacylation to peptide bond formation. The contributions have been studied in assays related to translation over a period that spans at least three decades. In this review, we follow the 2'- and 3'-OHs through the steps of translation and examine the involvement of these critical functional groups.
Figures
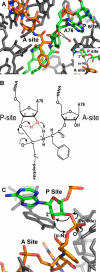
References
-
- Hill WE, Dahlberg A, Garrett RA, Moore PB, Schlessinger D, Warner JR. The Ribosome: Structure, Function and Evolution. American Society for Microbiology; Washington, D.C.: 1990.
-
- Nierhaus KH, Franceschi F, Subramanian AR, Erdmann VA, Wittmann-Liebold B. The Translational Apparatus: Structure, Function, Regulation, Evolution. Plenum Press; New York: 1994.
-
- Crick FH. On protein synthesis. Symp. Soc. Exp. Biol. 1958;12:138–163. - PubMed
-
- Lewin B. Genes VI. Oxford University Press; New York: 1997. pp. 151–241.
-
- Westhof E, Fritsch V. RNA folding: beyond Watson-Crick pairs. Structure Fold. Des. 2000;8:R55–65. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

