Polyadenylation of SV40 late pre-mRNA is dependent on phosphorylation of an essential component associated with the 3' end processing machinery
- PMID: 1668146
- PMCID: PMC5952190
Polyadenylation of SV40 late pre-mRNA is dependent on phosphorylation of an essential component associated with the 3' end processing machinery
Abstract
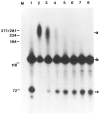
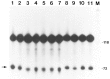
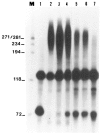
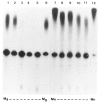
We investigated whether phosphorylation of the essential components involved in the 3' end processing of mRNAs was required for mRNA polyadenylation. The proteins in HeLa nuclear extract were dephosphorylated with alkaline phosphatase, which is known to remove the phosphate moieties from serine and tyrosine. The dephosphorylated extract was used for analyzing cleavage-dependent polyadenylation of SV40 late pre-mRNA. The phosphatase treatment of the extract completely blocked the polyadenylation reaction, whereas dephosphorylation of the extract did not inhibit the cleavage reaction. Since the cleavage depends upon functional integrity of the specificity factor, it is unlikely that the phosphorylated state of the latter factor is required for the 3' end processing. Sodium vanadate, a potent inhibitor of alkaline phosphatase, markedly reduced the inhibitory effect of the phosphatase on the polyadenylation reaction. Dephosphorylation of the extract also prevented formation of the polyadenylation-specific complex with pre-mRNA, whereas the cleavage-specific complexes were formed under this condition. The Mn-dependent polyadenylation, which is largely poly(A) extension reaction, was relatively resistant to the phosphatase treatment. These data indicate that phosphorylation of a key factor is essential for the 3' end processing of pre-mRNA, and suggest that the factor may be poly(A) polymerase.
Figures

Similar articles
-
Ara-ATP impairs 3'-end processing of pre-mRNAs by inhibiting both cleavage and polyadenylation.Nucleic Acids Res. 1991 Nov 11;19(21):5871-5. doi: 10.1093/nar/19.21.5871. Nucleic Acids Res. 1991. PMID: 1719481 Free PMC article.
-
A functionally redundant downstream sequence in SV40 late pre-mRNA is required for mRNA 3'-end formation and for assembly of a precleavage complex in vitro.J Biol Chem. 1988 Apr 25;263(12):5780-8. J Biol Chem. 1988. PMID: 2833517
-
Specific pre-cleavage and post-cleavage complexes involved in the formation of SV40 late mRNA 3' termini in vitro.EMBO J. 1987 Dec 20;6(13):4185-92. doi: 10.1002/j.1460-2075.1987.tb02765.x. EMBO J. 1987. PMID: 2832155 Free PMC article.
-
Polyadenylation of mRNA precursors.Biochim Biophys Acta. 1988 May 6;950(1):1-12. doi: 10.1016/0167-4781(88)90067-x. Biochim Biophys Acta. 1988. PMID: 2896017 Review. No abstract available.
-
mRNA polyadenylation and its coupling to other RNA processing reactions and to transcription.Curr Opin Cell Biol. 1999 Jun;11(3):352-7. doi: 10.1016/S0955-0674(99)80049-0. Curr Opin Cell Biol. 1999. PMID: 10395555 Review.
Cited by
-
GRSF-1: a poly(A)+ mRNA binding protein which interacts with a conserved G-rich element.Nucleic Acids Res. 1994 Jun 25;22(12):2334-43. doi: 10.1093/nar/22.12.2334. Nucleic Acids Res. 1994. PMID: 8036161 Free PMC article.
-
Finishing touches: post-translational modification of protein factors involved in mammalian pre-mRNA 3' end formation.Int J Biochem Cell Biol. 2008;40(11):2384-96. doi: 10.1016/j.biocel.2008.03.016. Epub 2008 Apr 1. Int J Biochem Cell Biol. 2008. PMID: 18468939 Free PMC article. Review.
-
Formation of mRNA 3' ends in eukaryotes: mechanism, regulation, and interrelationships with other steps in mRNA synthesis.Microbiol Mol Biol Rev. 1999 Jun;63(2):405-45. doi: 10.1128/MMBR.63.2.405-445.1999. Microbiol Mol Biol Rev. 1999. PMID: 10357856 Free PMC article. Review.
References
-
- Amster-Choder O., Houman F., and Wright A. (1989), Cell 58, 847–855. - PubMed
-
- Cantley L. C., Josephson L., Warner R., Yanagisawa M., Lechene C., and Guidotti G. (1977), J Biol Chem 252, 7421–7423. - PubMed
-
- Chen P. L., Scully P., Shew J. Y., Wang Y. J., and Lee W. H. (1989), Cell 58, 1193–1198. - PubMed
-
- Cherry J. R., Johnson T. R., Dollaid C., Shuster J. R., and Denis C. L. (1989), Cell 56, 409–419. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
