Niche-specific regulation of central metabolic pathways in a fungal pathogen
- PMID: 16681837
- PMCID: PMC1472618
- DOI: 10.1111/j.1462-5822.2005.00676.x
Niche-specific regulation of central metabolic pathways in a fungal pathogen
Abstract
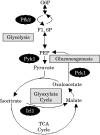
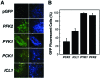
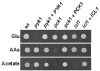
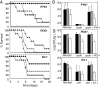
To establish an infection, the pathogen Candida albicans must assimilate carbon and grow in its mammalian host. This fungus assimilates six-carbon compounds via the glycolytic pathway, and two-carbon compounds via the glyoxylate cycle and gluconeogenesis. We address a paradox regarding the roles of these central metabolic pathways in C. albicans pathogenesis: the glyoxylate cycle is apparently required for virulence although glyoxylate cycle genes are repressed by glucose at concentrations present in the bloodstream. Using GFP fusions, we confirm that glyoxylate cycle and gluconeogenic genes in C. albicans are repressed by physiologically relevant concentrations of glucose, and show that these genes are inactive in the majority of fungal cells infecting the mouse kidney. However, these pathways are induced following phagocytosis by macrophages or neutrophils. In contrast, glycolytic genes are not induced following phagocytosis and are expressed in infected kidney. Mutations in all three pathways attenuate the virulence of this fungus, highlighting the importance of central carbon metabolism for the establishment of C. albicans infections. We conclude that C. albicans displays a metabolic program whereby the glyoxylate cycle and gluconeogenesis are activated early, when the pathogen is phagocytosed by host cells, while the subsequent progression of systemic disease is dependent upon glycolysis.
Figures


References
-
- Abi-Said D, Anaissie E, Uzun O, Raad I, Pinzcowski H, Vartivarian S. The epidemiology of hematogenous candidiasis caused by different Candida species. Clin Infect Dis. 1997;24:1122–1128. - PubMed
-
- Barelle CJ, Manson C, MacCallum D, Odds FC, Gow NAR, Brown AJP. GFP as a quantitative reporter of gene regulation in Candida albicans. Yeast. 2004;21:333–340. - PubMed
-
- Brown AJP. Integration of metabolism with virulence in Candida albicans. In: Brown AJP, editor. Fungal Genomics. Heidelberg: Mycota XIII, Springer-Verlag; 2005. pp. 185–203.
-
- Calderone RA. Candida and Candidiasis. Washington DC: American Society for Microbiology Press; 2002.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

