The miRNA-processing enzyme dicer is essential for the morphogenesis and maintenance of hair follicles
- PMID: 16682203
- PMCID: PMC2996092
- DOI: 10.1016/j.cub.2006.04.005
The miRNA-processing enzyme dicer is essential for the morphogenesis and maintenance of hair follicles
Abstract
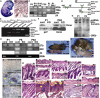
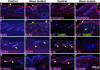
The discovery that microRNAs (miRNAs) play important roles in regulating gene expression via posttranscriptional repression has revealed a previously unsuspected mechanism controlling development and progenitor-cell function (reviewed in ); however, little is known of miRNA functions in mammalian organogenesis. Processing of miRNAs and their assembly into the RNA-induced silencing (RISC) complex requires the essential multifunctional enzyme Dicer . We found that Dicer mRNA and multiple miRNAs are expressed in mouse skin, suggesting roles in skin- and hair-follicle biology. In newborn mice carrying an epidermal-specific Dicer deletion, hair follicles were stunted and hypoproliferative. Hair-shaft and inner-root-sheath differentiation was initiated, but the mutant hair follicles were misoriented and expression of the key signaling molecules Shh and Notch1 was lost by postnatal day 7. At this stage, hair-follicle dermal papillae were observed to evaginate, forming highly unusual structures within the basal epidermis. Normal hair shafts were not produced in the Dicer mutant, and the follicles lacked stem cell markers and degenerated. In contrast to decreased follicular proliferation, the epidermis became hyperproliferative. These results reveal critical roles for Dicer in the skin and implicate miRNAs in key aspects of epidermal and hair-follicle development and function.
Figures


References
-
- He L, Hannon GJ. MicroRNAs: Small RNAs with a big role in gene regulation. Nat. Rev. Genet. 2004;5:522–531. - PubMed
-
- Bartel DP. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. - PubMed
-
- Millar SE. Molecular mechanisms regulating hair follicle development. J. Invest. Dermatol. 2002;118:216–225. - PubMed
-
- Levy V, Lindon C, Harfe BD, Morgan BA. Distinct stem cell populations regenerate the follicle and interfollicular epidermis. Dev. Cell. 2005;9:855–861. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

