Reversible post-translational modification of proteins by nitrated fatty acids in vivo
- PMID: 16682416
- PMCID: PMC2169497
- DOI: 10.1074/jbc.M602814200
Reversible post-translational modification of proteins by nitrated fatty acids in vivo
Abstract
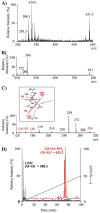
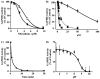
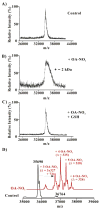
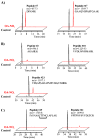
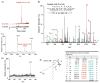
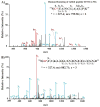
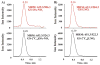
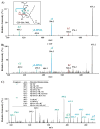
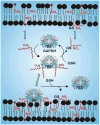
Nitric oxide ((*)NO)-derived reactive species nitrate unsaturated fatty acids, yielding nitroalkene derivatives, including the clinically abundant nitrated oleic and linoleic acids. The olefinic nitro group renders these derivatives electrophilic at the carbon beta to the nitro group, thus competent for Michael addition reactions with cysteine and histidine. By using chromatographic and mass spectrometric approaches, we characterized this reactivity by using in vitro reaction systems, and we demonstrated that nitroalkene-protein and GSH adducts are present in vivo under basal conditions in healthy human red cells. Nitro-linoleic acid (9-, 10-, 12-, and 13-nitro-9,12-octadecadienoic acids) (m/z 324.2) and nitro-oleic acid (9- and 10-nitro-9-octadecaenoic acids) (m/z 326.2) reacted with GSH (m/z 306.1), yielding adducts with m/z of 631.3 and 633.3, respectively. At physiological concentrations, nitroalkenes inhibited glyceraldehyde-3-phosphate dehydrogenase (GAPDH), which contains a critical catalytic Cys (Cys-149). GAPDH inhibition displayed an IC(50) of approximately 3 microM for both nitroalkenes, an IC(50) equivalent to the potent thiol oxidant peroxynitrite (ONOO(-)) and an IC(50) 30-fold less than H(2)O(2), indicating that nitroalkenes are potent thiol-reactive species. Liquid chromatography-mass spectrometry analysis revealed covalent adducts between fatty acid nitroalkene derivatives and GAPDH, including at the catalytic Cys-149. Liquid chromatography-mass spectrometry-based proteomic analysis of human red cells confirmed that nitroalkenes readily undergo covalent, thiol-reversible post-translational modification of nucleophilic amino acids in GSH and GAPDH in vivo. The adduction of GAPDH and GSH by nitroalkenes significantly increased the hydrophobicity of these molecules, both inducing translocation to membranes and suggesting why these abundant derivatives had not been detected previously via traditional high pressure liquid chromatography analysis. The occurrence of these electrophilic nitroalkylation reactions in vivo indicates that this reversible post-translational protein modification represents a new pathway for redox regulation of enzyme function, cell signaling, and protein trafficking.
Figures

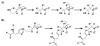
References
-
- Rubbo H, Darley-Usmar V, Freeman BA. Chem Res Toxicol. 1996;9:809–820. - PubMed
-
- Schopfer FJ, Baker PR, Giles G, Chumley P, Batthyany C, Crawford J, Patel RP, Hogg N, Branchaud BP, Lancaster JR, Jr, Freeman BA. J Biol Chem. 2005;280:19289–19297. - PubMed
-
- Napolitano A, Camera E, Picardo M, d'Ischia M. J Org Chem. 2000;65:4853–4860. - PubMed
-
- O'Donnell VB. Antioxid Redox Signal. 2003;5:195–203. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

