Alu RNP and Alu RNA regulate translation initiation in vitro
- PMID: 16682445
- PMCID: PMC1458521
- DOI: 10.1093/nar/gkl246
Alu RNP and Alu RNA regulate translation initiation in vitro
Abstract
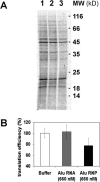
Alu elements are the most abundant repetitive elements in the human genome; they emerged from the signal recognition particle RNA gene and are composed of two related but distinct monomers (left and right arms). Alu RNAs transcribed from these elements are present at low levels at normal cell growth but various stress conditions increase their abundance. Alu RNAs are known to bind the cognate proteins SRP9/14. We purified synthetic Alu RNP, composed of Alu RNA in complex with SRP9/14, and investigated the effects of Alu RNPs and naked Alu RNA on protein translation. We found that the dimeric Alu RNP and the monomeric left and right Alu RNPs have a general dose-dependent inhibitory effect on protein translation. In the absence of SRP9/14, Alu RNA has a stimulatory effect on all reporter mRNAs. The unstable structure of sRight RNA suggests that the differential activities of Alu RNP and Alu RNA may be explained by conformational changes in the RNA. We demonstrate that Alu RNPs and Alu RNAs do not stably associate with ribosomes during translation and, based on the analysis of polysome profiles and synchronized translation, we show that Alu RNP and Alu RNA regulate translation at the level of initiation.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

