Lymphocyte recruitment into the aortic wall before and during development of atherosclerosis is partially L-selectin dependent
- PMID: 16682495
- PMCID: PMC2121208
- DOI: 10.1084/jem.20052205
Lymphocyte recruitment into the aortic wall before and during development of atherosclerosis is partially L-selectin dependent
Abstract
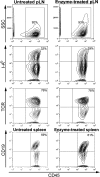
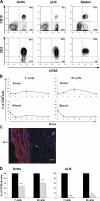
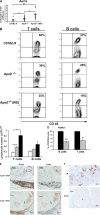
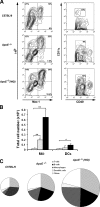
Atherosclerosis is an inflammatory disease of large arteries. Flow cytometry of aortic cell suspensions showed that B and T lymphocytes and some macrophages and dendritic cells are already present in the adventitia of normal/noninflamed mouse aortas. Adoptively transferred lymphocytes constitutively homed to the aorta and resided within the adventitia up to 7 d after transfer. Lymphocyte trafficking into normal/noninflamed or atherosclerosis-prone aortas was partially L-selectin dependent. Antigen-activated dendritic cells induced increased T lymphocyte proliferation within the aorta 72 h after adoptive transfer. During progression of atherosclerosis in apolipoprotein-E-deficient mice, the total number of macrophages, T cells, and dendritic cells, but not B cells, increased significantly. This alteration in immune cell composition was accompanied by the formation of tertiary lymphoid tissue in the adventitia of atherosclerotic aortas. These results demonstrate that lymphocytes already reside within the normal/noninflamed aorta before the onset atherosclerosis as a consequence of constitutive trafficking. Atherosclerosis induces the recruitment of macrophages and dendritic cells that support antigen presentation.
Figures


References
-
- Libby, P. 2002. Inflammation in atherosclerosis. Nature. 420:868–874. - PubMed
-
- Ross, R. 1999. Atherosclerosis–an inflammatory disease. N. Engl. J. Med. 340:115–126. - PubMed
-
- Hansson, G.K. 2005. Inflammation, atherosclerosis, and coronary artery disease. N. Engl. J. Med. 352:1685–1695. - PubMed
-
- Jonasson, L., J. Holm, O. Skalli, G. Bondjers, and G.K. Hansson. 1986. Regional accumulations of T cells, macrophages, and smooth muscle cells in the human atherosclerotic plaque. Arteriosclerosis. 6:131–138. - PubMed
-
- Buono, C., H. Pang, Y. Uchida, P. Libby, A.H. Sharpe, and A.H. Lichtman. 2004. B7-1/B7-2 costimulation regulates plaque antigen-specific T-cell responses and atherogenesis in low-density lipoprotein receptor-deficient mice. Circulation. 109:2009–2015. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

