Pannexin 1 in erythrocytes: function without a gap
- PMID: 16682648
- PMCID: PMC1472500
- DOI: 10.1073/pnas.0601037103
Pannexin 1 in erythrocytes: function without a gap
Abstract
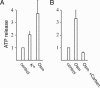
ATP is a widely used extracellular signaling molecule. The mechanism of ATP release from cells is presently unresolved and may be either vesicular or channel-mediated. Erythrocytes release ATP in response to low oxygen or to shear stress. In the absence of vesicles, the release has to be through channels. Erythrocytes do not form gap junctions. Yet, here we show with immunohistochemical and electrophysiological data that erythrocytes express the gap junction protein pannexin 1. This protein, in addition to forming gap junction channels in paired oocytes, can also form a mechanosensitive and ATP-permeable channel in the nonjunctional plasma membrane. Consistent with a role of pannexin 1 as an ATP release channel, ATP release by erythrocytes was attenuated by the gap junction blocker carbenoxolone. Furthermore, under conditions of ATP release, erythrocytes took up fluorescent tracer molecules permeant to gap junction channels.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures


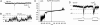

References
-
- Panchin Y., Kelmanson I., Matz M., Lukyanov K., Usman N., Lukyanov S. Curr. Biol. 2000;10:R473–R474. - PubMed
-
- Bao L., Locovei S., Dahl G. FEBS Lett. 2004;572:65–68. - PubMed
-
- Burnstock G., Knight G. E. Int. Rev. Cytol. 2004;240:31–304. - PubMed
-
- Bal-Price A., Moneer Z., Brown G. C. Glia. 2002;40:312–323. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

