Endocannabinoids potently protect the newborn brain against AMPA-kainate receptor-mediated excitotoxic damage
- PMID: 16682966
- PMCID: PMC1751782
- DOI: 10.1038/sj.bjp.0706755
Endocannabinoids potently protect the newborn brain against AMPA-kainate receptor-mediated excitotoxic damage
Abstract
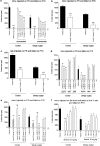
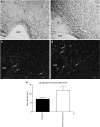
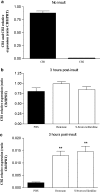
Brain lesions induced in newborn mice or rats by the glutamatergic agonists ibotenate (acting on NMDA and metabotropic receptors) or S-bromowillardiine (acting on AMPA-kainate receptors) mimic some aspects of white matter cysts and transcortical necrosis observed in human perinatal brain damage associated with cerebral palsy. Exogenous and endogenous cannabinoids have received increasing attention as potential neuroprotective agents in a number of neurodegenerative disorders of the adult. One recent study showed neuroprotection by the cannabinoid agonist WIN-55212 in a newborn rat model of acute severe asphyxia. The present study was designed to assess the neuroprotective effects of the endogenous cannabinoid anandamide using a well-defined rodent model of neonatal excitotoxic brain lesions. In this model, anandamide provided dose-dependent and long-lasting protection of developing white matter and cortical plate reducing the size of lesions induced by S-bromowillardiine. Anandamide had only marginal neuroprotective effect against ibotenate-induced cortical grey matter lesions. Anandamide-induced neuroprotection against AMPA-kainate receptor-mediated brain lesions were blocked by a CB1 antagonist but not by a CB2 antagonist. Furthermore, anandamide effects were mimicked by a CB1 agonist but not by a CB2 agonist. Real-time PCR confirmed the expression of CB1 receptors, but not CB2 receptors, in the untreated newborn neocortex. Finally, neuroprotective effects of anandamide in white matter involved increased survival of preoligodendrocytes and better preservation of myelination. The present study provides experimental support for the role of endocannabinoids as a candidate therapy for excitotoxic perinatal brain lesions.
Figures


References
-
- ACARIN L., GONZALEZ B., HIDALGO J., CASTRO A.J., CASTELLANO B. Primary cortical glial reaction versus secondary thalamic glial response in the excitotoxically injured young brain: astroglial response and metallothionein expression. Neuroscience. 1999;92:827–839. - PubMed
-
- ANTONELLI T., TOMASINI M.C., TATTOLI M., CASSANO T., TANGANELLI S., FINETTI S., MAZZONI E., TRABACE L., STEARDO L., CUOMO V., FERRARO L. Prenatal exposure to the CB1 receptor agonist WIN 55,212-2 causes learning disruption associated with impaired cortical NMDA receptor function and emotional reactivity changes in rat offspring. Cereb. Cortex. 2005;15:2013–2020. - PubMed
-
- BAKER D., PRYCE G., GIOVANNONI G., THOMPSON A.J. The therapeutic potential of cannabis. Lancet. Neurol. 2003;2:291–298. - PubMed
-
- BARKS J.D., SILVERSTEIN F.S. Excitatory amino acids contribute to the pathogenesis of perinatal hypoxic-ischemic brain injury. Brain. Pathol. 1992;2:235–243. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

