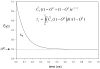
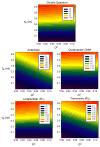
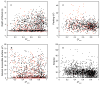
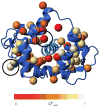
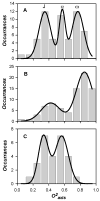
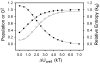
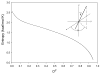
Figure 8
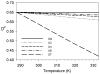
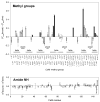
Temperature dependence of side chain methyl
parameters of calmodulin in complex with the smMLCKp peptide. Panel a, the methyl
parameters determined for the beta methyl groups of alanines: A10 (●), A15 (○), A46 (▼), A73 (▽), A88 (■), A102 (□), A103 (◆), A128 (◇), and A147 (▲). Panel b, the methyl
parameters determined for the gamma methyl groups of threonines: T26 (●), T29 (○), T34 (▼), T70 (▽), T79 (■), T110 (□), and T146 (◆). Panel c, the methyl
parameters determined for the gamma methyl groups of valines: V35-γR (●), V35-γS (○), V55-γR (▼), V55-γS (△), V108-γR (■), V108-γS (□), and V121-γR (◆), V136-γR (◇), V142-γR (▲), and V142-γS. Panel d, the methyl
parameters determined for isoleucine-γ methyls: I27 (●), I52 (○), I63 (▼), I85 (△), I125 (■), and I130 (□). Panel e, the methyl
parameters determined for isoleucine-δ methyls: I9 (●), I27 (○), I52 (▼), I63 (▽), I85 (■), I100 (□), I125 (◆), and I130 (◇). Panel f, the methyl
parameters determined for leucine-δ methyls: L18δS (●), L39δR (○), L39δS (▼), L69δR (▽), L105δR (■), L112δS (□), and L116δR (◆). Panel g, the methyl
parameters determined for methionine-δ methyls: M71 (●), M72 (○), M76 (▼), M109 (▽), M124 (■), M144 (□), and M145 (◆). In many cases the error bars are less than dimensions of the symbol. Reproduced with permission from Biochemistry 2002, 41, 13814–25. Copyright 2002 American Chemical Society.