Single-molecule fluorescence studies of protein folding and conformational dynamics
- PMID: 16683755
- PMCID: PMC2569857
- DOI: 10.1021/cr0404343
Single-molecule fluorescence studies of protein folding and conformational dynamics
Figures
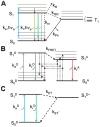
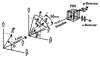
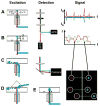
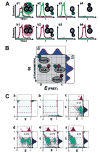
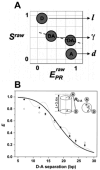
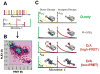
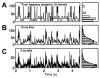
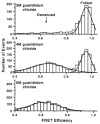
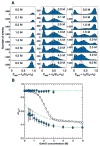
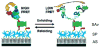
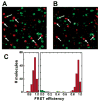
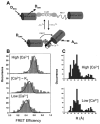
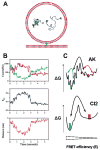
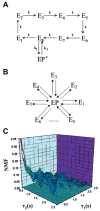
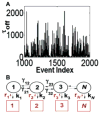
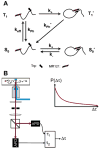
References
-
- Basché T, Moerner WE, Orrit M, Wild UP, editors. Single-Molecule Optical Detection, Imaging and Spectroscopy. VCH; Weinheim: 1997.
-
- Nie S, Zare RN. Annu Rev Biophys Biomol Struct. 1997;26:567. - PubMed
-
- Xie XS, Trautman JK. Annu Rev Phys Chem. 1998;49:441. - PubMed
-
- Moerner WE, Orrit M. Science. 1999;283:1670. - PubMed
-
- Weiss S. Science. 1999;283:1676. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

