Immune protection of nonhuman primates against Ebola virus with single low-dose adenovirus vectors encoding modified GPs
- PMID: 16683867
- PMCID: PMC1459482
- DOI: 10.1371/journal.pmed.0030177
Immune protection of nonhuman primates against Ebola virus with single low-dose adenovirus vectors encoding modified GPs
Abstract
Background: Ebola virus causes a hemorrhagic fever syndrome that is associated with high mortality in humans. In the absence of effective therapies for Ebola virus infection, the development of a vaccine becomes an important strategy to contain outbreaks. Immunization with DNA and/or replication-defective adenoviral vectors (rAd) encoding the Ebola glycoprotein (GP) and nucleoprotein (NP) has been previously shown to confer specific protective immunity in nonhuman primates. GP can exert cytopathic effects on transfected cells in vitro, and multiple GP forms have been identified in nature, raising the question of which would be optimal for a human vaccine.
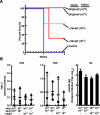
Methods and findings: To address this question, we have explored the efficacy of mutant GPs from multiple Ebola virus strains with reduced in vitro cytopathicity and analyzed their protective effects in the primate challenge model, with or without NP. Deletion of the GP transmembrane domain eliminated in vitro cytopathicity but reduced its protective efficacy by at least one order of magnitude. In contrast, a point mutation was identified that abolished this cytopathicity but retained immunogenicity and conferred immune protection in the absence of NP. The minimal effective rAd dose was established at 10(10) particles, two logs lower than that used previously.
Conclusions: Expression of specific GPs alone vectored by rAd are sufficient to confer protection against lethal challenge in a relevant nonhuman primate model. Elimination of NP from the vaccine and dose reductions to 10(10) rAd particles do not diminish protection and simplify the vaccine, providing the basis for selection of a human vaccine candidate.
Conflict of interest statement
Figures

References
-
- Baize S, Leroy EM, Georges-Courbot MC, Capron M, Lansoud-Soukate J, et al. Defective humoral responses and extensive intravascular apoptosis are associated with fatal outcome in Ebola virus-infected patients. Nat Med. 1999;5:423–426. - PubMed
-
- Sullivan NJ, Sanchez A, Rollin PE, Yang ZY, Nabel GJ. Development of a preventive vaccine for Ebola virus infection in primates. Nature. 2000;408:605–609. - PubMed
-
- Xu L, Sanchez A, Yang Z, Zaki SR, Nabel EG, et al. Immunization for Ebola virus infection. Nat Med. 1998;4:37–42. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

