Pim-1 kinase phosphorylates RUNX family transcription factors and enhances their activity
- PMID: 16684349
- PMCID: PMC1473194
- DOI: 10.1186/1471-2121-7-21
Pim-1 kinase phosphorylates RUNX family transcription factors and enhances their activity
Abstract
Background: The pim family genes encode oncogenic serine/threonine kinases which in hematopoietic cells have been implicated in cytokine-dependent signaling as well as in lymphomagenesis, especially in cooperation with other oncogenes such as myc, bcl-2 or Runx family genes. The Runx genes encode alpha-subunits of heterodimeric transcription factors which regulate cell proliferation and differentiation in various tissues during development and which can become leukemogenic upon aberrant expression.
Results: Here we have identified novel protein-protein interactions between the Pim-1 kinase and the RUNX family transcription factors. Using the yeast two-hybrid system, we were able to show that the C-terminal part of human RUNX3 associates with Pim-1. This result was confirmed in cell culture, where full-length murine Runx1 and Runx3 both coprecipitated and colocalized with Pim-1. Furthermore, catalytically active Pim-1 kinase was able to phosphorylate Runx1 and Runx3 proteins and enhance the transactivation activity of Runx1 in a dose-dependent fashion.
Conclusion: Altogether, our results suggest that mammalian RUNX family transcription factors are novel binding partners and substrates for the Pim-1 kinase, which may be able to regulate their activities during normal hematopoiesis as well as in leukemogenesis.
Figures
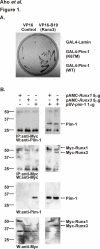
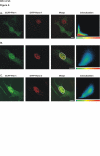
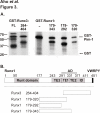

References
-
- Acton D, Domen J, Jacobs H, Vlaar M, Korsmeyer S, Berns A. Collaboration of pim -1 and bcl -2 in lymphomagenesis. Curr Top Microbiol Immunol. 1992;182:293–298. - PubMed
-
- Dautry F, Weil D, Yu J, Dautry-Varsat A. Regulation of pim and myb mRNA accumulation by interleukin 2 and interleukin 3 in murine hematopoietic cell lines. J Biol Chem. 1988;263:17615–17620. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

