Muscarinic receptor signaling in the pathophysiology of asthma and COPD
- PMID: 16684353
- PMCID: PMC1479816
- DOI: 10.1186/1465-9921-7-73
Muscarinic receptor signaling in the pathophysiology of asthma and COPD
Abstract
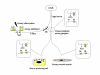
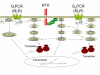
Anticholinergics are widely used for the treatment of COPD, and to a lesser extent for asthma. Primarily used as bronchodilators, they reverse the action of vagally derived acetylcholine on airway smooth muscle contraction. Recent novel studies suggest that the effects of anticholinergics likely extend far beyond inducing bronchodilation, as the novel anticholinergic drug tiotropium bromide can effectively inhibit accelerated decline of lung function in COPD patients. Vagal tone is increased in airway inflammation associated with asthma and COPD; this results from exaggerated acetylcholine release and enhanced expression of downstream signaling components in airway smooth muscle. Vagally derived acetylcholine also regulates mucus production in the airways. A number of recent research papers also indicate that acetylcholine, acting through muscarinic receptors, may in part regulate pathological changes associated with airway remodeling. Muscarinic receptor signalling regulates airway smooth muscle thickening and differentiation, both in vitro and in vivo. Furthermore, acetylcholine and its synthesizing enzyme, choline acetyl transferase (ChAT), are ubiquitously expressed throughout the airways. Most notably epithelial cells and inflammatory cells generate acetylcholine, and express functional muscarinic receptors. Interestingly, recent work indicates the expression and function of muscarinic receptors on neutrophils is increased in COPD. Considering the potential broad role for endogenous acetylcholine in airway biology, this review summarizes established and novel aspects of muscarinic receptor signaling in relation to the pathophysiology and treatment of asthma and COPD.
Figures

References
-
- Gross NJ, Skorodin MS. Role of the parasympathetic system in airway obstruction due to emphysema. N Engl J Med. 1984;311:421–425. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

