Dental composites based on amorphous calcium phosphate - resin composition/physicochemical properties study
- PMID: 16684798
- PMCID: PMC2424213
- DOI: 10.1177/0885328206064823
Dental composites based on amorphous calcium phosphate - resin composition/physicochemical properties study
Abstract
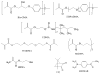
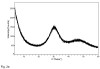
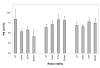
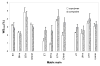
This study explores how the resin composition/structure affects the physicochemical properties of copolymers and their amorphous calcium phosphate (ACP)-filled composites. A series of photo-polymerizable binary and ternary matrices are formulated utilizing 2,2-bis[ p-(2(')-hydroxy-3(')methacryloxypropoxy)phenyl]propane, 2,2-bis[ p-(2(')-methacryloxypropoxy)phenyl]-propane (EBPADMA), or a urethane dimethacrylate as base monomers, and triethylene glycol dimethacrylate or hexamethylene dimethacrylate (HmDMA) with or without 2-hydroxyethyl methacrylate (HEMA) as diluent monomer. Unfilled copolymers and composites filled with 40% by mass zirconia-hybridized ACP are evaluated for biaxial flexure strength (BFS), degree of conversion (DC), mineral ion release, polymerization shrinkage (PS), and water sorption (WS). The average DC values are 82-94% and 74-91% for copolymers and composites, respectively. Unrelated to the resin composition, the PS values of composites are up to 8.4 vol. % and the BFS values of wet composite specimens are on average 51 +/- 8 MPa. The maximum WS values attained in copolymers and composites reach 4.8 mass%. Inclusion of hydrophobic HmDMA monomer in the matrices significantly reduces the WS. The levels of Ca and PO(4) released from all types of composites are significantly above the minimum necessary for the re-deposition of apatite to occur. Elevated Ca, and to a lesser extent PO(4) release, is observed in HEMA-containing, ternary EBPADMA formulations. Further resin reformulations may be needed to improve the PS of composite specimens. Poor dispersion of ;as-synthesized' ACP within the composite contributes to their inferior mechanical performance.
Figures






References
-
- Skrtic D, Stansbury JW, Antonucci JM. Volumetric Contraction and Methacrylate Conversion in Photo-polymerized Amorphous Calcium Phosphate/methacrylate Composites. Biomaterials. 2003;24:2443–2449. - PubMed
-
- Skrtic D, Antonucci JM, Eanes ED, Eichmiller FC, Schumacher GE. Physicochemical Evaluation of Bioactive Polymeric Composites Based on Hybrid Amorphous Calcium Phosphates. J Biomed Mat Res (Appl Biomater) 2000;53:381–391. - PubMed
-
- Skrtic D, Hailer AW, Takagi S, Antonucci JM, Eanes ED. Quantitative Assessment of the Efficacy of Amorphous Calcium Phosphate/methacrylate Composites in Remineralizing Caries-like Lesions Artificially Produced in Bovine Enamel. J Dent Res. 1996;75(9):1679–1686. - PubMed
-
- Skrtic D, Antonucci JM, Eanes ED, Eidelman N. Dental Composites Based on Hybrid and Surface-modified Amorphous Calcium Phosphates – A FTIR Microspectroscopic Study. Biomaterials. 2004;25:1141–1150. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

