A biochemical rationale for the anticancer effects of Hsp90 inhibitors: slow, tight binding inhibition by geldanamycin and its analogues
- PMID: 16684877
- PMCID: PMC1458618
- DOI: 10.1073/pnas.0602650103
A biochemical rationale for the anticancer effects of Hsp90 inhibitors: slow, tight binding inhibition by geldanamycin and its analogues
Abstract
Heat shock protein (Hsp)90 is emerging as an important therapeutic target for the treatment of cancer. Two analogues of the Hsp90 inhibitor geldanamycin are currently in clinical trials. Geldanamycin (GA) and its analogues have been reported to bind purified Hsp90 with low micromolar potency, in stark contrast to their low nanomolar antiproliferative activity in cell culture and their potent antitumor activity in animal models. Several models have been proposed to account for the approximately 100-fold-greater potency in cell culture, including that GA analogues bind with greater affinity to a five-protein Hsp90 complex than to Hsp90 alone. We have determined that GA and the fluorescent analogue BODIPY-GA (BDGA) both demonstrate slow, tight binding to purified Hsp90. BDGA, used to characterize the kinetics of ligand-Hsp90 interactions, was found to bind Hsp90alpha with k(off) = 2.5 x 10(-3) min(-1), t(1/2) = 4.6 h, and Ki* = 10 nM. It was found that BDGA binds to a functional multiprotein Hsp90 complex with kinetics and affinity identical to that of Hsp90 alone. Also, BDGA binds to Hsp90 from multiple cell lysates in a time-dependent manner with similar kinetics. Therefore, our results indicate that the high potency of GA in cell culture and in vivo can be accounted for by its time-dependent, tight binding to Hsp90 alone. In the broader context, these studies highlight the essentiality of detailed biochemical characterization of drug-target interactions for the effective translation of in vitro pharmacology to cellular and in vivo efficacy.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures
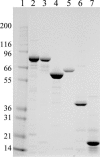
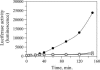


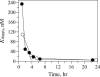
References
-
- Pratt W. B. Proc. Soc. Exp. Biol. Med. 1998;217:420–434. - PubMed
-
- Pearl L. H., Prodromou C. Curr. Opin. Struct. Biol. 2000;10:46–51. - PubMed
-
- Kosano H., Stensgard B., Charlesworth M. C., McMahon N., Toft D. J. Biol. Chem. 1998;273:32973–32979. - PubMed
-
- Isaacs J. S. Expert. Opin. Investig. Drugs. 2005;14:569–589. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

