The clinical benefit of pegylated liposomal doxorubicin in patients with metastatic breast cancer previously treated with conventional anthracyclines: a multicentre phase II trial
- PMID: 16685267
- PMCID: PMC2361305
- DOI: 10.1038/sj.bjc.6603158
The clinical benefit of pegylated liposomal doxorubicin in patients with metastatic breast cancer previously treated with conventional anthracyclines: a multicentre phase II trial
Abstract
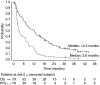
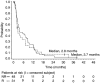
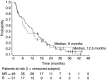
This study evaluates the clinical benefit of pegylated liposomal doxorubicin (PLD) in patients with metastatic breast cancer (MBC), previously treated with conventional anthracyclines. Seventy-nine women with MBC previously treated with anthracyclines received PLD 50 mg m(-2) every 4 weeks. All patients were previously treated with chemotherapy and 30% of patients had > or =3 prior chemotherapies for metastatic disease. Patients were considered anthracycline resistant when they had disease progression on anthracycline therapy for MBC or within 6 months of adjuvant therapy. The overall clinical benefit rate (objective response+stable disease > or =24 weeks) was 24% (16.1% in patients with documented anthracycline resistance vs 29% in patients classified as having non-anthracycline-resistant disease). There was no difference with respect to the clinical benefit between patients who received PLD >12 months and those who received PLD < or =12 months since last anthracycline treatment for metastatic disease (clinical benefit 25 vs 24.1%, respectively). Median time to progression and overall survival were 3.6 and 12.3 months, respectively. The median duration of response was 12 months, and the median time to progression in patients with stable disease (any) was 9.5 months. Fourteen patients (17.7%) had a prolonged clinical benefit lasting > or =12 months. In conclusion, PLD was associated with an evident clinical benefit in anthracycline-pretreated patients with MBC.
Figures
References
-
- Albain K, Elledge R, Gradishar WJ, Hayes DF, Rowinsky E, Hudis C, Pusztai L, Tripathy D, Modi S, Rubi S (2002) Open-label phase II, multicenter trial of ZD1839 (‘Iressa’) in patients with advanced breast cancer. Breast Cancer Res Treat 76: S33, (abstract 20)
-
- Blum JL, Dieras V, Lo Russo PM, Horton J, Rutman O, Buzdar A, Osterwalder B (2001) Multicenter, Phase II study of capecitabine in taxane-pretreated metastatic breast carcinoma patients. Cancer 92: 1759–1768 - PubMed
-
- Gabizon A, Martin F (1997) Polyethylene glycol-coated (pegylated) liposomal doxorubicin: rationale for use in solid tumours. Drugs 54: 15–21 - PubMed
-
- Gabizon A, Shmeeda H, Barenholz Y (2003) Pharmacokinetics of pegylated liposomal doxorubicin: review of animal and human studies. Clin Pharmacokinet 42: 419–436 - PubMed
-
- Keller AM, Mennel RG, Georgoulias VA, Nabholtz JM, Erazo A, Lluch A, Vogel CL, Kaufmann M, von Minckwitz G, Henderson IC, Mellars L, Alland L, Tendler C (2004) Randomized phase III trial of pegylated liposomal doxorubicin vs vinorelbine or mitomycin C plus vinblastine in women with taxane-refractory advanced breast cancer. J Clin Oncol 22: 3893–3901 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

