The role of sodium channel current in modulating transmural dispersion of repolarization and arrhythmogenesis
- PMID: 16686686
- PMCID: PMC1474079
- DOI: 10.1111/j.1540-8167.2006.00388.x
The role of sodium channel current in modulating transmural dispersion of repolarization and arrhythmogenesis
Abstract
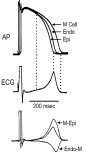
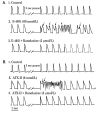
Ventricular myocardium in larger mammals is composed of three distinct cell types: epicardial, M, and endocardial cells. Epicardial and M cell, but not endocardial cell, action potentials have a prominent I(to)-mediated notch. M cells are distinguished from the other cell types in that they display a smaller I(Ks), but a larger late I(Na) and I(Na-Ca). These ionic differences may account for the longer action potential duration (APD) and steeper APD-rate relationship of the M cell. The difference in the time course of repolarization of phase 1 and phase 3 contributes to the inscription of the electrocardiographic J wave and T wave, respectively. These repolarization gradients are modulated by electrotonic interactions, [K(+)](o), and agents or mutations that alter net repolarizing current. An increase in late I(Na), as occurring under a variety of pathophysiological states or in response to certain toxins, leads to a preferential prolongation of the M cell action potential, thus prolonging the QT interval and increasing transmural dispersion of repolarization (TDR), which underlies the development of torsade de pointes (TdP) arrhythmias. Agents that reduce late I(Na) are effective in reducing TDR and suppressing TdP. A reduction in peak I(Na) or an increase in net repolarizing current in the early phases of the action potential can lead to a preferential abbreviation of the action potential of epicardium in the right ventricle, and thus the development of a large TDR, phase 2 reentry, and polymorphic ventricular tachycardia associated with the Brugada syndrome.
Figures



References
-
- Antzelevitch C, Dumaine R. Electrical heterogeneity in the heart: Physiological, pharmacological and clinical implications. In: Page E, Fozzard HA, Solaro RJ, editors. Handbook of Physiology. Section 2 The Cardiovascular System. Oxford University Press; New York: 2001. pp. 654–692.
-
- Di Diego JM,, Sun ZQ. Antzelevitch C: Ito and action potential notch are smaller in left vs. right canine ventricular epicardium. Am J Physiol. 1996;271:H548–H561. - PubMed
-
- Volders PG, Sipido KR, Carmeliet E, Spatjens RL, Wellens HJ, Vos MA. Repolarizing K+ currents ITO1 and IKs are larger in right than left canine ventricular midmyocardium. Circulation. 1999;99(2):206–210. - PubMed
-
- Sicouri S, Antzelevitch C. A subpopulation of cells with unique electrophysiological properties in the deep subepicardium of the canine ventricle. The M cell. Circ Res. 1991;68:1729–1741. - PubMed
-
- Antzelevitch C, Shimizu W, Yan GX, Sicouri S, Weissenburger J, Nesterenko VV, Burashnikov A, Di Diego JM,, Saffitz JE, Thomas GP. The M cell: Its contribution to the ECG and to normal and abnormal electrical function of the heart. J Cardiovasc Electrophysiol. 1999;10(8):1124–1152. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

