The effect of engineered disulfide bonds on the stability of Drosophila melanogaster acetylcholinesterase
- PMID: 16686937
- PMCID: PMC1481510
- DOI: 10.1186/1471-2091-7-12
The effect of engineered disulfide bonds on the stability of Drosophila melanogaster acetylcholinesterase
Abstract
Background: Acetylcholinesterase is irreversibly inhibited by organophosphate and carbamate insecticides allowing its use in biosensors for detection of these insecticides. Drosophila acetylcholinesterase is the most sensitive enzyme known and has been improved by in vitro mutagenesis. However, its stability has to be improved for extensive utilization.
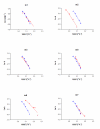
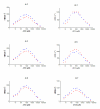
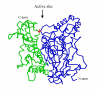
Results: To create a disulfide bond that could increase the stability of the Drosophila melanogaster acetylcholinesterase, we selected seven positions taking into account first the distance between Cbeta of two residues, in which newly introduced cysteines will form the new disulfide bond and second the conservation of the residues in the cholinesterase family. Most disulfide bonds tested did not increase and even decreased the stability of the protein. However, one engineered disulfide bridge, I327C/D375C showed significant stability increase toward denaturation by temperature (170 fold at 50 degrees C), urea, organic solvent and provided resistance to protease degradation. The new disulfide bridge links the N-terminal domain (first 356 aa) to the C-terminal domain. The quantities produced by this mutant were the same as in wild-type flies.
Conclusion: Addition of a disulfide bridge may either stabilize or unstabilize proteins. One bond out of the 7 tested provided significant stabilisation.
Figures
References
-
- Marty JL, Sode K, Karube I. Biosensor for detection of organophosphate and carbamate insecticides. Electroanalysis. 1992;4:801–803. doi: 10.1002/elan.1140040217. - DOI
-
- Bachmann TT, Leca B, Vilatte F, Marty JL, Fournier D, Schmid RD. Improved multianalyte detection of organophosphates and carbamates with disposable multielectrode biosensors using recombinant mutants of Drosophila acetylcholinesterase and artificial neural networks. Biosens Bioelectron. 2000;15:193–201. doi: 10.1016/S0956-5663(00)00055-5. - DOI - PubMed
-
- Payne CS, Saeed M, Wolfe AD. Ligand stabilization of cholinesterases. Biochim Biophys Acta. 1989;999:46–51. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

