The placental cholinergic system: localization to the cytotrophoblast and modulation of nitric oxide
- PMID: 16686954
- PMCID: PMC1481520
- DOI: 10.1186/1478-811X-4-4
The placental cholinergic system: localization to the cytotrophoblast and modulation of nitric oxide
Abstract
Background: The human placenta, a non-neuronal tissue, contains an active cholinergic system comprised of acetylcholine (ACh), choline acetyltransferase (ChAT), acetylcholinesterase (AChE), and high affinity muscarinic receptors. The cell(s) of origin of placental ACh and its role in trophoblast function has not been defined. These studies were performed to define the cellular location of ACh synthesis (ChAT) in the human placenta and to begin studying its functional role.
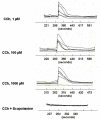
Results: Using immunohistochemical techniques, ChAT was observed primarily within the cytotrophoblasts of preterm placentae as well as some mesenchymal elements. Similar intense immunostaining of the cytotrophoblast was observed for endothelium-derived nitric oxide synthase (eNOS) suggesting that ACh may interact with nitric oxide (NO)-dependent signaling pathways. The ability of carbamylcholine (CCh), an ACh analogue, to stimulate a rise in intracellular Ca++ and NO production in trophoblasts was therefore tested using the BeWob30 choriocarcinoma cell as a model system. First, CCh significantly increased intracellular calcium as assessed by fluorescence microscopy. We then examined the ability of CCh to stimulate NO production by measuring total nitrite/nitrate production in conditioned media using chemiluminescence-based analysis. CCh, alone, had no effect on NO production. However, CCh increased measurable NO approximately 100% in the presence of 10 nM estradiol. This stimulatory effect was inhibited by 1 (micro)M scopolamine suggesting mediation via muscarinic receptors. Estradiol, alone, had no effect on total NO or eNOS protein or mRNA.
Conclusion: These data demonstrate that placental ChAT localizes to the cytotrophoblast and some mesenchymal cells in human placenta. It further suggests that ACh acts via muscarinic receptors on the trophoblast cell membrane to modulate NO in an estrogen-dependent manner.
Figures


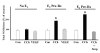
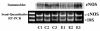
References
LinkOut - more resources
Full Text Sources

