Co-contraction and passive forces facilitate load compensation of aimed limb movements
- PMID: 16687491
- PMCID: PMC6674257
- DOI: 10.1523/JNEUROSCI.0161-06.2006
Co-contraction and passive forces facilitate load compensation of aimed limb movements
Abstract
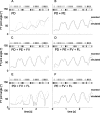
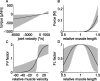
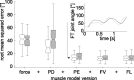
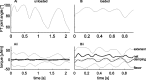
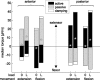
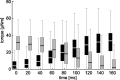
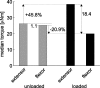
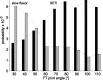
Vertebrates and arthropods are both capable of load compensation during aimed limb movements, such as reaching and grooming. We measured the kinematics and activity of individual motoneurons in loaded and unloaded leg movements in an insect. To evaluate the role of active and passive musculoskeletal properties in aiming and load compensation, we used a neuromechanical model of the femur-tibia joint that transformed measured extensor and flexor motoneuron spikes into joint kinematics. The model comprises three steps: first, an activation dynamics module that determines the time course of isometric force; second, a pair of antagonistic muscle models that determine the joint torque; and third, a forward dynamics simulation that calculates the movement of the limb. The muscles were modeled in five variants, differing in the presence or absence of force-length-velocity characteristics of the contractile element, a parallel passive elastic element, and passive joint damping. Each variant was optimized to yield the best simulation of measured behavior. Passive muscle force and viscous joint damping were sufficient and necessary to simulate the observed movements. Elastic or damping properties of the active contractile element could not replace passive elements. Passive elastic forces were similar in magnitude to active forces caused by muscle contraction, generating substantial joint stiffness. Antagonistic muscles co-contract, although there was no motoneuronal coactivation, because of slow dynamics of muscle activation. We quantified how co-contraction simplified load compensation by demonstrating that a small variation of the motoneuronal input caused a large change in joint torque.
Figures




References
-
- Ahn A, Full R (2002). A motor and a brake: two leg extensor muscles acting at the same joint manage energy differently in a running insect. J Exp Biol 205:379–389. - PubMed
-
- Bässler U, Stein W (1996). Contributions of structure and innervation pattern of the stick insect extensor tibiae muscle to the filter characteristics of the muscle-joint system. J Exp Biol 199:2185–2198. - PubMed
-
- Bennet-Clark H (1975). The energetics of the jump of the locust Schistocerca gregaria. J Exp Biol 63:53–83. - PubMed
-
- Blackburn L (2005). Behavioural and physiological differences in the motor systems of solitarious and gregarious locusts. PhD thesis University of Cambridge.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
