Depolarizing GABAergic conductances regulate the balance of excitation to inhibition in the developing retinotectal circuit in vivo
- PMID: 16687503
- PMCID: PMC6674233
- DOI: 10.1523/JNEUROSCI.0319-06.2006
Depolarizing GABAergic conductances regulate the balance of excitation to inhibition in the developing retinotectal circuit in vivo
Abstract
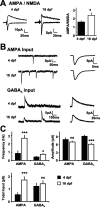
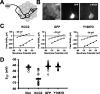
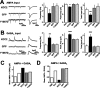
Neurotransmission during development regulates synaptic maturation in neural circuits, but the contribution of different neurotransmitter systems is unclear. We investigated the role of GABAA receptor-mediated Cl- conductances in the development of synaptic responses in the Xenopus visual system. Intracellular Cl- concentration ([Cl-]i) was found to be high in immature tectal neurons and then falls over a period of several weeks. GABAergic synapses are present at early stages of tectal development and, when activated by optic nerve stimulation or visual stimuli, induce sustained depolarizing Cl- conductances that facilitate retinotectal transmission by NMDA receptors. To test whether depolarizing GABAergic inputs cooperate with NMDA receptors during activity-dependent maturation of glutamatergic synapses, we prematurely reduced [Cl-]i in tectal neurons in vivo by expressing the Cl- transporter KCC2. This blocked the normal developmental increase in AMPA receptor-mediated retinotectal transmission and increased GABAergic synaptic input to tectal neurons. Therefore, depolarizing GABAergic transmission plays a pivotal role in the maturation of excitatory transmission and controls the balance of excitation and inhibition in the developing retinotectal circuit.
Figures






References
-
- Aamodt SM, Shi J, Colonnese MT, Veras W, Constantine-Paton M (2000). Chronic NMDA exposure accelerates development of GABAergic inhibition in the superior colliculus. J Neurophysiol 83:1580–1591. - PubMed
-
- Aizenman CD, Akerman CJ, Jensen KR, Cline HT (2003). Visually driven regulation of intrinsic neuronal excitability improves stimulus detection in vivo. Neuron 39:831–842. - PubMed
-
- Artola A, Brocher S, Singer W (1990). Different voltage-dependent thresholds for inducing long-term depression and long-term potentiation in slices of rat visual cortex. Nature 347:69–72. - PubMed
-
- Ben-Ari Y (2002). Excitatory actions of GABA during development: the nature of the nurture. Nat Rev Neurosci 3:728–739. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
