Spectrins and ankyrinB constitute a specialized paranodal cytoskeleton
- PMID: 16687515
- PMCID: PMC6674250
- DOI: 10.1523/JNEUROSCI.0425-06.2006
Spectrins and ankyrinB constitute a specialized paranodal cytoskeleton
Abstract
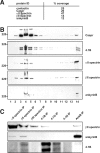
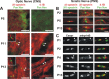
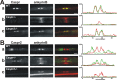
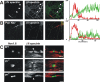
Paranodal junctions of myelinated nerve fibers are important for saltatory conduction and function as paracellular and membrane protein diffusion barriers flanking nodes of Ranvier. The formation of these specialized axoglial contacts depends on the presence of three cell adhesion molecules: neurofascin 155 on the glial membrane and a complex of Caspr and contactin on the axon. We isolated axonal and glial membranes highly enriched in these paranodal proteins and then used mass spectrometry to identify additional proteins associated with the paranodal axoglial junction. This strategy led to the identification of three novel components of the paranodal cytoskeleton: ankyrinB, alphaII spectrin, and betaII spectrin. Biochemical and immunohistochemical analyses revealed that these proteins associate with protein 4.1B in a macromolecular complex that is concentrated at central and peripheral paranodal junctions in the adult and during early myelination. Furthermore, we show that the paranodal localization of ankyrinB is disrupted in Caspr-null mice with aberrant paranodal junctions, demonstrating that paranodal neuron-glia interactions regulate the organization of the underlying cytoskeleton. In contrast, genetic disruption of the juxtaparanodal protein Caspr2 or the nodal cytoskeletal protein betaIV spectrin did not alter the paranodal cytoskeleton. Our results demonstrate that the paranodal junction contains specialized cytoskeletal components that may be important to stabilize axon-glia interactions and contribute to the membrane protein diffusion barrier found at paranodes.
Figures



Similar articles
-
Localization of Caspr2 in myelinated nerves depends on axon-glia interactions and the generation of barriers along the axon.J Neurosci. 2001 Oct 1;21(19):7568-75. doi: 10.1523/JNEUROSCI.21-19-07568.2001. J Neurosci. 2001. PMID: 11567047 Free PMC article.
-
Glial βII Spectrin Contributes to Paranode Formation and Maintenance.J Neurosci. 2018 Jul 4;38(27):6063-6075. doi: 10.1523/JNEUROSCI.3647-17.2018. Epub 2018 May 31. J Neurosci. 2018. PMID: 29853631 Free PMC article.
-
An αII Spectrin-Based Cytoskeleton Protects Large-Diameter Myelinated Axons from Degeneration.J Neurosci. 2017 Nov 22;37(47):11323-11334. doi: 10.1523/JNEUROSCI.2113-17.2017. Epub 2017 Oct 16. J Neurosci. 2017. PMID: 29038243 Free PMC article.
-
Submembranous cytoskeletons stabilize nodes of Ranvier.Exp Neurol. 2016 Sep;283(Pt B):446-51. doi: 10.1016/j.expneurol.2015.11.012. Epub 2016 Jan 14. Exp Neurol. 2016. PMID: 26775177 Review.
-
Glial regulation of the axonal membrane at nodes of Ranvier.Curr Opin Neurobiol. 2006 Oct;16(5):508-14. doi: 10.1016/j.conb.2006.08.003. Epub 2006 Sep 1. Curr Opin Neurobiol. 2006. PMID: 16945520 Review.
Cited by
-
Glial Contributions to Neural Function and Disease.Mol Cell Proteomics. 2016 Feb;15(2):355-61. doi: 10.1074/mcp.R115.053744. Epub 2015 Sep 4. Mol Cell Proteomics. 2016. PMID: 26342039 Free PMC article. Review.
-
Regulation and dysregulation of axon infrastructure by myelinating glia.J Cell Biol. 2017 Dec 4;216(12):3903-3916. doi: 10.1083/jcb.201702150. Epub 2017 Nov 7. J Cell Biol. 2017. PMID: 29114067 Free PMC article. Review.
-
LGR5 is a proneural factor and is regulated by OLIG2 in glioma stem-like cells.Cell Mol Neurobiol. 2013 Aug;33(6):851-65. doi: 10.1007/s10571-013-9951-6. Epub 2013 Jun 21. Cell Mol Neurobiol. 2013. PMID: 23793848 Free PMC article.
-
Disruption of the axon initial segment cytoskeleton is a new mechanism for neuronal injury.J Neurosci. 2009 Oct 21;29(42):13242-54. doi: 10.1523/JNEUROSCI.3376-09.2009. J Neurosci. 2009. PMID: 19846712 Free PMC article.
-
Glial ankyrins facilitate paranodal axoglial junction assembly.Nat Neurosci. 2014 Dec;17(12):1673-81. doi: 10.1038/nn.3858. Epub 2014 Nov 2. Nat Neurosci. 2014. PMID: 25362471 Free PMC article.
References
-
- Arroyo EJ, Xu T, Poliak S, Watson M, Peles E, Scherer SS (2001). Internodal specializations of myelinated axons in the central nervous system. Cell Tissue Res 305:53–66. - PubMed
-
- Becker PS, Schwartz MA, Morrow JS, Lux SE (1990). Radiolabel-transfer cross-linking demonstrates that protein 4.1 binds to the N-terminal region of beta spectrin and to actin in binary interactions. Eur J Biochem 193:827–836. - PubMed
-
- Bennett V, Baines AJ (2001). Spectrin and ankyrin-based pathways: metazoan inventions for integrating cells into tissues. Physiol Rev 81:1353–1392. - PubMed
-
- Berghs S, Aggujaro D, Dirkx R, Maksimova E, Stabach P, Hermel JM, Zhang JP, Philbrick W, Slepnev V, Ort T, Solimena M (2000). betaIV spectrin, a new spectrin localized at axon initial segments and nodes of ranvier in the central and peripheral nervous system. J Cell Biol 151:985–1002. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
