Parkinson's disease brain mitochondrial complex I has oxidatively damaged subunits and is functionally impaired and misassembled
- PMID: 16687518
- PMCID: PMC6674236
- DOI: 10.1523/JNEUROSCI.0984-06.2006
Parkinson's disease brain mitochondrial complex I has oxidatively damaged subunits and is functionally impaired and misassembled
Abstract
Loss of mitochondrial complex I catalytic activity in the electron transport chain (ETC) is found in multiple tissues from individuals with sporadic Parkinson's disease (PD) and is a property of some PD model neurotoxins. Using special ETC subunit-specific and complex I immunocapture antibodies directed against the entire complex I macroassembly, we quantified ETC proteins and protein oxidation of complex I subunits in brain mitochondria from 10 PD and 12 age-matched control (CTL) samples. We measured nicotinamide adenine dinucleotide (NADH)-driven electron transfer rates through complex I and correlated these with complex I subunit oxidation levels and reductions of its 8 kDa subunit. PD brain complex I shows 11% increase in ND6, 34% decrease in its 8 kDa subunit and contains 47% more protein carbonyls localized to catalytic subunits coded for by mitochondrial and nuclear genomes We found no changes in levels of ETC proteins from complexes II-V. Oxidative damage patterns to PD complex I are reproduced by incubation of CTL brain mitochondria with NADH in the presence of rotenone but not by exogenous oxidant. NADH-driven electron transfer rates through complex I inversely correlate with complex I protein oxidation status and positively correlate with reduction in PD 8 kDa subunit. Reduced complex I function in PD brain mitochondria appears to arise from oxidation of its catalytic subunits from internal processes, not from external oxidative stress, and correlates with complex I misassembly. This complex I auto-oxidation may derive from abnormalities in mitochondrial or nuclear encoded subunits, complex I assembly factors, rotenone-like complex I toxins, or some combination.
Figures
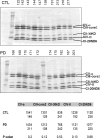
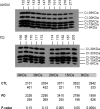


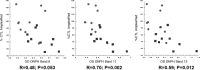


References
-
- Antonicka H, Ogilvie I, Taivassalo T, Anitori RP, Haller RG, Vissing J, Kennaway NG, Shoubridge EA (2003). Identification and characterization of a common set of complex I assembly intermediates in mitochondria from patients with complex I deficiency. J Biol Chem 278:43081–43088. - PubMed
-
- Chen YR, Chen CL, Zhang L, Green-Church KB, Zweier JL (2005). Superoxide generation from mitochondrial NADH dehydrogenase induces self-inactivation with specific protein radical formation. J Biol Chem 280:37339–37348. - PubMed
-
- Genova ML, Pich MM, Bernacchia A, Bianchi C, Biondi A, Bovina C, Falasca AI, Formiggini G, Castelli GP, Lenaz G (2004). The mitochondrial production of reactive oxygen species in relation to aging and pathology. Ann NY Acad Sci 1011:86–100. - PubMed
-
- Greenamyre JT, Betarbet R, Sherer TB (2003). The rotenone model of Parkinson's disease: genes, environment and mitochondria. Parkinsonism Relat Disord 9:[Suppl 2], S59–S64. - PubMed
-
- Gu M, Cooper JM, Taanman JW, Schapira AH (1998). Mitochondrial DNA transmission of the mitochondrial defect in Parkinson's disease. Ann Neurol 44:177–186. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
