In vitro studies of the neuroform microstent using transparent human intracranial arteries
- PMID: 16687559
- PMCID: PMC7975717
In vitro studies of the neuroform microstent using transparent human intracranial arteries
Abstract
For better understanding of relevant morphology and mechanics, direct visualization of a Neuroform microstent (NFM) within an actual human intracranial artery is essential. Twelve NFM were deployed into 8 various segments of formaldehyde-fixed cadaver intracranial arteries. The arteries were then dehydrated and cleared in methyl salicylate to create transparency. The morphology of NFM was studied by digital macro-photography with a back illumination system. The possible limitations and important findings of the NFM were discussed.
Figures


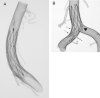



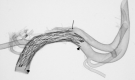

References
-
- Howington JU, Hanel RA, Harrigan MR, et al. The Neuroform stent, the first microcatheter-delivered stent for use in the intracranial circulation. Neurosurgery 2004;54:2–5 - PubMed
-
- Karino T, Motomiya M. Flow visualization in isolated transparent natural blood vessels. Biorheology 1983;20:119–27 - PubMed
-
- Lylyk P, Cohen JE, Ceratto R, et al. Endovascular reconstruction of intracranial arteries by stent placement and combined techniques. J Neurosurg 2002;97:1306–13 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
