The docking protein Cas links tyrosine phosphorylation signaling to elongation of cerebellar granule cell axons
- PMID: 16687575
- PMCID: PMC1483050
- DOI: 10.1091/mbc.e05-12-1122
The docking protein Cas links tyrosine phosphorylation signaling to elongation of cerebellar granule cell axons
Abstract
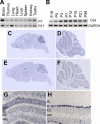
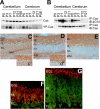
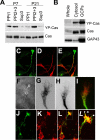
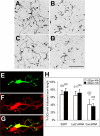
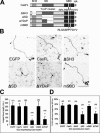
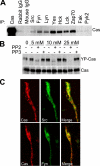
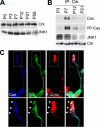
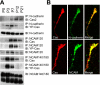
Crk-associated substrate (Cas) is a tyrosine-phosphorylated docking protein that is indispensable for the regulation of the actin cytoskeletal organization and cell migration in fibroblasts. The function of Cas in neurons, however, is poorly understood. Here we report that Cas is dominantly enriched in the brain, especially the cerebellum, of postnatal mice. During cerebellar development, Cas is highly tyrosine phosphorylated and is concentrated in the neurites and growth cones of granule cells. Cas coimmunoprecipitates with Src family protein tyrosine kinases, Crk, and cell adhesion molecules and colocalizes with these proteins in granule cells. The axon extension of granule cells is inhibited by either RNA interference knockdown of Cas or overexpression of the Cas mutant lacking the YDxP motifs, which are tyrosine phosphorylated and thereby interact with Crk. These findings demonstrate that Cas acts as a key scaffold that links the proteins associated with tyrosine phosphorylation signaling pathways to the granule cell axon elongation.
Figures
Similar articles
-
The SH2 domain protein Shep1 regulates the in vivo signaling function of the scaffolding protein Cas.Cell Signal. 2010 Nov;22(11):1745-52. doi: 10.1016/j.cellsig.2010.06.015. Epub 2010 Jul 24. Cell Signal. 2010. PMID: 20603213 Free PMC article.
-
PKC-dependent activation of FAK and src induces tyrosine phosphorylation of Cas and formation of Cas-Crk complexes.Exp Cell Res. 2001 Apr 1;264(2):296-306. doi: 10.1006/excr.2000.5137. Exp Cell Res. 2001. PMID: 11262186
-
Src regulates phorbol 12-myristate 13-acetate-activated PKC-induced migration via Cas/Crk/Rac1 signaling pathway in glioblastoma cells.Int J Mol Med. 2007 Oct;20(4):511-9. Int J Mol Med. 2007. PMID: 17786281
-
New concepts regarding focal adhesion kinase promotion of cell migration and proliferation.J Cell Biochem. 2006 Sep 1;99(1):35-52. doi: 10.1002/jcb.20956. J Cell Biochem. 2006. PMID: 16823799 Review.
-
p130 Crk-associated substrate (CAS) in vascular smooth muscle.J Cardiovasc Pharmacol Ther. 2009 Jun;14(2):89-98. doi: 10.1177/1074248409333490. Epub 2009 Mar 27. J Cardiovasc Pharmacol Ther. 2009. PMID: 19329671 Free PMC article. Review.
Cited by
-
Netrin-1 attracts axons through FAK-dependent mechanotransduction.J Neurosci. 2012 Aug 22;32(34):11574-85. doi: 10.1523/JNEUROSCI.0999-12.2012. J Neurosci. 2012. PMID: 22915102 Free PMC article.
-
Ethanol inhibits neuronal differentiation by disrupting activity-dependent neuroprotective protein signaling.Proc Natl Acad Sci U S A. 2008 Dec 16;105(50):19962-7. doi: 10.1073/pnas.0807758105. Epub 2008 Dec 1. Proc Natl Acad Sci U S A. 2008. PMID: 19047645 Free PMC article.
-
Cas adaptor proteins organize the retinal ganglion cell layer downstream of integrin signaling.Neuron. 2014 Feb 19;81(4):779-86. doi: 10.1016/j.neuron.2014.01.036. Neuron. 2014. PMID: 24559672 Free PMC article.
-
Profiling, Bioinformatic, and Functional Data on the Developing Olfactory/GnRH System Reveal Cellular and Molecular Pathways Essential for This Process and Potentially Relevant for the Kallmann Syndrome.Front Endocrinol (Lausanne). 2013 Dec 31;4:203. doi: 10.3389/fendo.2013.00203. eCollection 2013. Front Endocrinol (Lausanne). 2013. PMID: 24427155 Free PMC article.
-
Developmental expression and differentiation-related neuron-specific splicing of metastasis suppressor 1 (Mtss1) in normal and transformed cerebellar cells.BMC Dev Biol. 2007 Oct 9;7:111. doi: 10.1186/1471-213X-7-111. BMC Dev Biol. 2007. PMID: 17925019 Free PMC article.
References
-
- Azuma K., Tanaka M., Uekita T., Inoue S., Yokota J., Ouchi Y., Sakai R. Tyrosine phosphorylation of paxillin affects the metastatic potential of human osteosarcoma. Oncogene. 2005;24:4754–4764. - PubMed
-
- Beggs H. E., Baragona S. C., Hemperly J. J., Maness P. F. NCAM140 interacts with the focal adhesion kinase p125(fak) and the SRC-related tyrosine kinase p59(fyn) J. Biol. Chem. 1997;272:8310–8319. - PubMed
-
- Bjorkblom B., Ostman N., Hongisto V., Komarovski V., Filen J. J., Nyman T. A., Kallunki T., Courtney M. J., Coffey E. T. Constitutively active cytoplasmic c-Jun N-terminal kinase 1 is a dominant regulator of dendritic architecture: role of microtubule-associated protein 2 as an effector. J. Neurosci. 2005;25:6350–6361. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

