Cell cycle-dependent roles for the FCH-domain protein Cdc15p in formation of the actomyosin ring in Schizosaccharomyces pombe
- PMID: 16687577
- PMCID: PMC1483054
- DOI: 10.1091/mbc.e05-11-1086
Cell cycle-dependent roles for the FCH-domain protein Cdc15p in formation of the actomyosin ring in Schizosaccharomyces pombe
Abstract
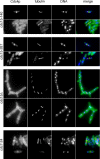
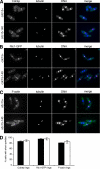
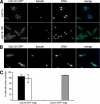
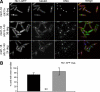
Cell division in the fission yeast Schizosaccharomyces pombe requires the formation and constriction of an actomyosin ring at the division site. The actomyosin ring is assembled in metaphase and anaphase A, is maintained throughout mitosis, and constricts after completion of anaphase. Maintenance of the actomyosin ring during late stages of mitosis depends on the septation initiation network (SIN), a signaling cascade that also regulates the deposition of the division septum. However, SIN is not active in metaphase and is not required for the initial assembly of the actomyosin ring early in mitosis. The FER/CIP4-homology (FCH) domain protein Cdc15p is a component of the actomyosin ring. Mutations in cdc15 lead to failure in cytokinesis and result in the formation of elongated, multinucleate cells without a division septum. Here we present evidence that the requirement of Cdc15p for actomyosin ring formation is dependent on the stage of mitosis. Although cdc15 mutants are competent to assemble actomyosin rings in metaphase, they are unable to maintain actomyosin rings late in mitosis when SIN is active. In the absence of functional Cdc15p, ring formation upon metaphase arrest depends on the anillin-like Mid1p. Interestingly, when cytokinesis is delayed due to perturbations to the division machinery, Cdc15p is maintained in a hypophosphorylated form. The dephosphorylation of Cdc15p, which occurs transiently in unperturbed cytokinesis, is partially dependent on the phosphatase Clp1p/Flp1p. This suggests a mechanism where both SIN and Clp1p/Flp1p contribute to maintenance of the actomyosin ring in late mitosis through Cdc15p, possibly by regulating its phosphorylation status.
Figures

Similar articles
-
The Clp1p/Flp1p phosphatase ensures completion of cytokinesis in response to minor perturbation of the cell division machinery in Schizosaccharomyces pombe.J Cell Sci. 2004 Aug 1;117(Pt 17):3897-910. doi: 10.1242/jcs.01244. Epub 2004 Jul 20. J Cell Sci. 2004. PMID: 15265986
-
Etd1p is a novel protein that links the SIN cascade with cytokinesis.EMBO J. 2005 Jul 6;24(13):2436-46. doi: 10.1038/sj.emboj.7600705. Epub 2005 Jun 2. EMBO J. 2005. PMID: 15933715 Free PMC article.
-
The 14-3-3 protein rad24p modulates function of the cdc14p family phosphatase clp1p/flp1p in fission yeast.Curr Biol. 2005 Aug 9;15(15):1376-83. doi: 10.1016/j.cub.2005.06.070. Curr Biol. 2005. PMID: 16085489
-
Overview of fission yeast septation.Cell Microbiol. 2016 Sep;18(9):1201-7. doi: 10.1111/cmi.12611. Epub 2016 Jun 1. Cell Microbiol. 2016. PMID: 27155541 Review.
-
Coordinating septum formation and the actomyosin ring during cytokinesis in Schizosaccharomyces pombe.Mol Microbiol. 2019 Dec;112(6):1645-1657. doi: 10.1111/mmi.14387. Epub 2019 Sep 30. Mol Microbiol. 2019. PMID: 31533197 Free PMC article. Review.
Cited by
-
The final cut: cell polarity meets cytokinesis at the bud neck in S. cerevisiae.Cell Mol Life Sci. 2016 Aug;73(16):3115-36. doi: 10.1007/s00018-016-2220-3. Epub 2016 Apr 16. Cell Mol Life Sci. 2016. PMID: 27085703 Free PMC article. Review.
-
Of bars and rings: Hof1-dependent cytokinesis in multiseptated hyphae of Ashbya gossypii.Mol Cell Biol. 2009 Feb;29(3):771-83. doi: 10.1128/MCB.01150-08. Epub 2008 Nov 24. Mol Cell Biol. 2009. PMID: 19029253 Free PMC article.
-
DYRK kinase Pom1 drives F-BAR protein Cdc15 from the membrane to promote medial division.Mol Biol Cell. 2020 Apr 15;31(9):917-929. doi: 10.1091/mbc.E20-01-0026. Epub 2020 Feb 26. Mol Biol Cell. 2020. PMID: 32101481 Free PMC article.
-
The F-BAR Cdc15 promotes contractile ring formation through the direct recruitment of the formin Cdc12.J Cell Biol. 2015 Feb 16;208(4):391-9. doi: 10.1083/jcb.201411097. J Cell Biol. 2015. PMID: 25688133 Free PMC article.
-
Model of myosin node aggregation into a contractile ring: the effect of local alignment.J Phys Condens Matter. 2011 Sep 21;23(37):374103. doi: 10.1088/0953-8984/23/37/374103. Epub 2011 Aug 23. J Phys Condens Matter. 2011. PMID: 21862839 Free PMC article.
References
-
- Arai R., Mabuchi I. F-actin ring formation and the role of F-actin cables in the fission yeast Schizosaccharomyces pombe. J. Cell Sci. 2002;115:887–898. - PubMed
-
- Balasubramanian M. K., Bi E., Glotzer M. Comparative analysis of cytokinesis in budding yeast, fission yeast and animal cells. Curr. Biol. 2004;14:R806–R818. - PubMed
-
- Balasubramanian M. K., McCollum D., Gould K. L. Cytokinesis in fission yeast Schizosaccharomyces pombe. Methods Enzymol. 1997;283:494–506. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

