Aurora-B/AIM-1 regulates the dynamic behavior of HP1alpha at the G2-M transition
- PMID: 16687578
- PMCID: PMC1483052
- DOI: 10.1091/mbc.e05-09-0906
Aurora-B/AIM-1 regulates the dynamic behavior of HP1alpha at the G2-M transition
Abstract
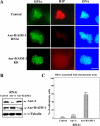
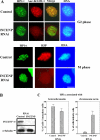
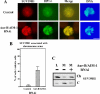
Heterochromatin protein 1 (HP1) plays an important role in heterochromatin formation and undergoes large-scale, progressive dissociation from heterochromatin in prophase cells. However, the mechanisms regulating the dynamic behavior of HP1 are poorly understood. In this study, the role of Aurora-B was investigated with respect to the dynamic behavior of HP1alpha. Mammalian Aurora-B, AIM-1, colocalizes with HP1alpha to the heterochromatin in G2. Depletion of Aurora-B/AIM-1 inhibited dissociation of HP1alpha from the chromosome arms at the G2-M transition. In addition, depletion of INCENP led to aberrant cellular localization of Aurora-B/AIM-1, but it did not affect heterochromatin targeting of HP1alpha. It was proposed in the binary switch hypothesis that phosphorylation of histone H3 at Ser-10 negatively regulates the binding of HP1alpha to the adjacent methylated Lys-9. However, Aurora-B/AIM-1-mediated phosphorylation of H3 induced dissociation of the HP1alpha chromodomain but not of the intact protein in vitro, indicating that the center and/or C-terminal domain of HP1alpha interferes with the effect of H3 phosphorylation on HP1alpha dissociation. Interestingly, Lys-9 methyltransferase SUV39H1 is abnormally localized together along the metaphase chromosome arms in Aurora-B/AIM-1-depleted cells. In conclusion, these results showed that Aurora-B/AIM-1 is necessary for regulated histone modifications involved in binding of HP1alpha by the N terminus of histone H3 during mitosis.
Figures
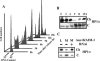

Similar articles
-
Regulation of HP1-chromatin binding by histone H3 methylation and phosphorylation.Nature. 2005 Dec 22;438(7071):1116-22. doi: 10.1038/nature04219. Epub 2005 Oct 12. Nature. 2005. PMID: 16222246
-
Histone H3 serine 10 phosphorylation by Aurora B causes HP1 dissociation from heterochromatin.Nature. 2005 Dec 22;438(7071):1176-80. doi: 10.1038/nature04254. Epub 2005 Oct 12. Nature. 2005. PMID: 16222244
-
Inhibitors of histone deacetylases alter kinetochore assembly by disrupting pericentromeric heterochromatin.Cell Cycle. 2005 May;4(5):717-26. doi: 10.4161/cc.4.5.1690. Epub 2005 May 28. Cell Cycle. 2005. PMID: 15846093
-
Beyond the histone tale: HP1α deregulation in breast cancer epigenetics.Cancer Biol Ther. 2015;16(2):189-200. doi: 10.1080/15384047.2014.1001277. Cancer Biol Ther. 2015. PMID: 25588111 Free PMC article. Review.
-
Role of chromosomal passenger complex in chromosome segregation and cytokinesis.Cell Struct Funct. 2001 Dec;26(6):653-7. doi: 10.1247/csf.26.653. Cell Struct Funct. 2001. PMID: 11942622 Review.
Cited by
-
Inhibition of histone H3-H4 chaperone pathways rescues C. elegans sterility by H2B loss.PLoS Genet. 2022 Jun 9;18(6):e1010223. doi: 10.1371/journal.pgen.1010223. eCollection 2022 Jun. PLoS Genet. 2022. PMID: 35679337 Free PMC article.
-
Cell division: control of the chromosomal passenger complex in time and space.Chromosoma. 2014 Mar;123(1-2):25-42. doi: 10.1007/s00412-013-0437-6. Epub 2013 Oct 3. Chromosoma. 2014. PMID: 24091645 Free PMC article. Review.
-
Chromatin modifiers in human disease: from functional roles to regulatory mechanisms.Mol Biomed. 2024 Apr 8;5(1):12. doi: 10.1186/s43556-024-00175-1. Mol Biomed. 2024. PMID: 38584203 Free PMC article. Review.
-
Structural role of RKS motifs in chromatin interactions: a molecular dynamics study of HP1 bound to a variably modified histone tail.Biophys J. 2012 Apr 18;102(8):1926-33. doi: 10.1016/j.bpj.2012.03.030. Biophys J. 2012. PMID: 22768949 Free PMC article.
-
SUV39H1 orchestrates temporal dynamics of centromeric methylation essential for faithful chromosome segregation in mitosis.J Mol Cell Biol. 2012 Oct;4(5):331-40. doi: 10.1093/jmcb/mjs023. Epub 2012 Jul 25. J Mol Cell Biol. 2012. PMID: 22831836 Free PMC article.
References
-
- Aagaard L., Schmid M., Warburton P., Jenuwein T. Mitotic phosphorylation of SUV39H1, a novel component of active centromeres, coincides with transient accumulation at mammalian centromeres. J. Cell Sci. 2000;113:817–829. - PubMed
-
- Adams R. R., Carmena M., Earnshaw W. C. Chromosomal passengers and the (aurora) ABCs of mitosis. Trends Cell Biol. 2001a;11:49–54. - PubMed
-
- Adams R. R., Wheatley S. P., Gouldsworthy A. M., Kandels-Lewis S. E., Carmena M., Smythe C., Gerloff D. L., Earnshaw W. C. INCENP binds the Aurora-related kinase AIRK2 and is required to target it to chromosomes, the central spindle and cleavage furrow. Curr. Biol. 2000;10:1075–1078. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

