A real-time PCR-based assay for detection of Wuchereria bancrofti DNA in blood and mosquitoes
- PMID: 16687688
- PMCID: PMC2196401
A real-time PCR-based assay for detection of Wuchereria bancrofti DNA in blood and mosquitoes
Abstract
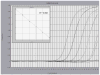
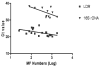
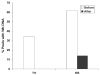
We developed and evaluated real-time polymerase chain reaction (PCR) assays for detecting Wuchereria bancrofti DNA in human blood and in mosquitoes. An assay based on detection of the W. bancrofti "LDR" repeat DNA sequence was more sensitive than an assay for Wolbachia 16S rDNA. The LDR-based assay was sensitive for detecting microfilarial DNA on dried membrane filters or on filter paper. We also compared real-time PCR with conventional PCR (C-PCR) for detecting W. bancrofti DNA in mosquito samples collected in endemic areas in Egypt and Papua New Guinea. Although the two methods had comparable sensitivity for detecting filarial DNA in reference samples, real-time PCR was more sensitive than C-PCR in practice with field samples. Other advantages of real-time PCR include its high-throughput capacity and decreased risk of cross-contamination between test samples. We believe that real-time PCR has great potential as a tool for monitoring progress in large-scale filariasis elimination programs.
Figures

Similar articles
-
Combined detection of Brugia malayi and Wuchereria bancrofti using single PCR.Acta Trop. 2005 Mar;93(3):233-7. doi: 10.1016/j.actatropica.2005.01.001. Acta Trop. 2005. PMID: 15715996
-
Xenomonitoring of different filarial nematodes using single and multiplex PCR in mosquitoes from Assiut Governorate, Egypt.Korean J Parasitol. 2015 Feb;53(1):77-83. doi: 10.3347/kjp.2015.53.1.77. Epub 2015 Feb 27. Korean J Parasitol. 2015. PMID: 25748712 Free PMC article.
-
A polymerase chain reaction-based assay for detection of Wuchereria bancrofti in human blood and Culex pipiens.Trans R Soc Trop Med Hyg. 1997 Mar-Apr;91(2):156-60. doi: 10.1016/s0035-9203(97)90205-4. Trans R Soc Trop Med Hyg. 1997. PMID: 9196756
-
Prospects, drawbacks and future needs of xenomonitoring for the endpoint evaluation of lymphatic filariasis elimination programs in Africa.Trans R Soc Trop Med Hyg. 2016 Feb;110(2):90-7. doi: 10.1093/trstmh/trv104. Trans R Soc Trop Med Hyg. 2016. PMID: 26822601 Review.
-
DNA-based diagnosis of lymphatic filariasis.Southeast Asian J Trop Med Public Health. 2009 Sep;40(5):904-13. Southeast Asian J Trop Med Public Health. 2009. PMID: 19842372 Review.
Cited by
-
Positivity of Antigen Tests Used for Diagnosis of Lymphatic Filariasis in Individuals Without Wuchereria bancrofti Infection But with High Loa loa Microfilaremia.Am J Trop Med Hyg. 2016 Dec 7;95(6):1417-1423. doi: 10.4269/ajtmh.16-0547. Epub 2016 Oct 10. Am J Trop Med Hyg. 2016. PMID: 27729568 Free PMC article.
-
Diagnosis of brugian filariasis by loop-mediated isothermal amplification.PLoS Negl Trop Dis. 2012;6(12):e1948. doi: 10.1371/journal.pntd.0001948. Epub 2012 Dec 13. PLoS Negl Trop Dis. 2012. PMID: 23272258 Free PMC article.
-
Monitoring lymphatic filariasis interventions: Adult mosquito sampling, and improved PCR - based pool screening method for Wuchereria bancrofti infection in Anopheles mosquitoes.Filaria J. 2007 Nov 29;6:13. doi: 10.1186/1475-2883-6-13. Filaria J. 2007. PMID: 18047647 Free PMC article.
-
Multi-centric evaluation of a stage-specific reverse transcriptase-polymerase chain reaction assay as a xenomonitoring tool for the detection of infective (L3) stage Wuchereria bancrofti in vectors.Indian J Med Res. 2021 Jul;154(1):132-140. doi: 10.4103/ijmr.IJMR_713_19. Indian J Med Res. 2021. PMID: 34782539 Free PMC article.
-
Filarial antigenemia and Loa loa night blood microfilaremia in an area without bancroftian filariasis in the Democratic Republic of Congo.Am J Trop Med Hyg. 2014 Dec;91(6):1142-1148. doi: 10.4269/ajtmh.14-0358. Epub 2014 Sep 15. Am J Trop Med Hyg. 2014. PMID: 25223938 Free PMC article.
References
-
- Grove DI, Warren KS, Mahmoud AA. Algorithms in the diagnosis and management of exotic diseases. VI. The filariases. J Infect Dis. 1975;132:340–352. - PubMed
-
- Weil GJ, Lammie PJ, Weiss N. The ICT filariasis test: a rapid-format antigen test for diagnosis of bancroftian filariasis. Parasitol Today. 1997;13:401–404. - PubMed
-
- Williams S, Laney S, Bierwert L, Saunders L, Boakye D, Fischer P, Goodman D, Helmy H, Hoti S, Vasuki V, Lammie P, Plichart C, Ramzy R, Ottesen E. Development and standardization of a rapid PCR-based method for the detection of Wuchereria bancrofti in mosquitoes for xenomonitoring the human prevalence of bancroftian filariasis. Ann Trop Med Parasitol. 2002;96:S41–S46. - PubMed
-
- Ramzy R, Farid H, Kamal H, Ibrahim G, Morsy Z, Faris R, Weil G. A polymerase chain reaction-based assay for detection of Wuchereria bancrofti in human blood and Culex pipiens. Trans Roy Soc Trop Med Hyg. 1997;91:156–160. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
