Molecular chaperones of the Hsp110 family act as nucleotide exchange factors of Hsp70s
- PMID: 16688212
- PMCID: PMC1478182
- DOI: 10.1038/sj.emboj.7601138
Molecular chaperones of the Hsp110 family act as nucleotide exchange factors of Hsp70s
Abstract
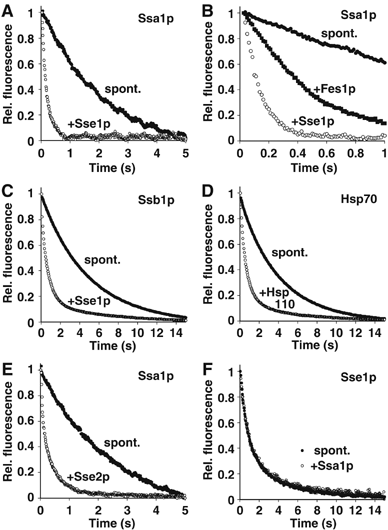
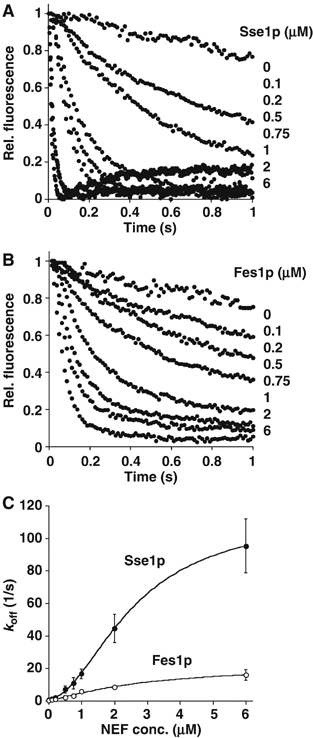
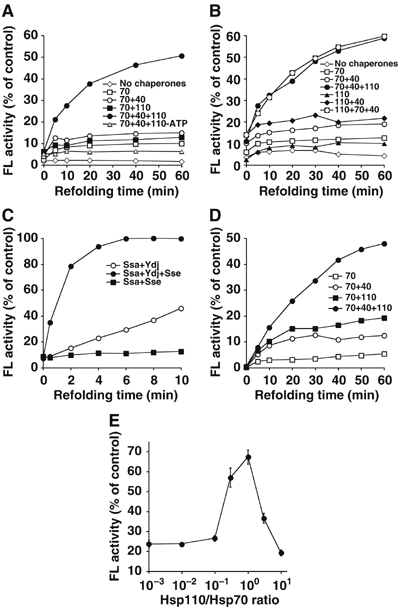
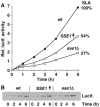
Hsp70 molecular chaperones function in protein folding in a manner dependent on regulation by co-chaperones. Hsp40s increase the low intrinsic ATPase activity of Hsp70, and nucleotide exchange factors (NEFs) remove ADP after ATP hydrolysis, enabling a new Hsp70 interaction cycle with non-native protein substrate. Here, we show that members of the Hsp70-related Hsp110 family cooperate with Hsp70 in protein folding in the eukaryotic cytosol. Mammalian Hsp110 and the yeast homologues Sse1p/2p catalyze efficient nucleotide exchange on Hsp70 and its orthologue in Saccharomyces cerevisiae, Ssa1p, respectively. Moreover, Sse1p has the same effect on Ssb1p, a ribosome-associated isoform of Hsp70 in yeast. Mutational analysis revealed that the N-terminal ATPase domain and the ultimate C-terminus of Sse1p are required for nucleotide exchange activity. The Hsp110 homologues significantly increase the rate and yield of Hsp70-mediated re-folding of thermally denatured firefly luciferase in vitro. Similarly, deletion of SSE1 causes a firefly luciferase folding defect in yeast cells under heat stress in vivo. Our data indicate that Hsp110 proteins are important components of the eukaryotic Hsp70 machinery of protein folding.
Figures


References
-
- Agashe VR, Guha S, Chang H-C, Genevaux P, Hayer-Hartl M, Stemp M, Georgopoulos C, Hartl FU, Barral JM (2004) Function of trigger factor and DnaK in multidomain protein folding: increase in yield at the expense of folding speed. Cell 117: 199–209 - PubMed
-
- Anttonen AK, Mahjneh I, Hamalainen RH, Lagier-Tourenne C, Kopra O, Waris L, Anttonen M, Joensuu T, Kalimo H, Paetau A, Tranebjaerg L, Chaigne D, Koenig M, Eeg-Olofsson O, Udd B, Somer M, Somer H, Lehesjoki AE (2005) The gene disrupted in Marinesco–Sjogren syndrome encodes SIL1, an HSPA5 cochaperone. Nat Genet 37: 1309–1311 - PubMed
-
- Brehmer D, Rudiger S, Gassler CS, Klostermeier D, Packschies L, Reinstein J, Mayer MP, Bukau B (2001) Tuning of chaperone activity of Hsp70 proteins by modulation of nucleotide exchange. Nat Struct Biol 8: 427–432 - PubMed
-
- Bukau B, Horwich AL (1998) The Hsp70 and Hsp60 chaperone machines. Cell 92: 351–366 - PubMed
-
- Chen X, Easton D, Oh HJ, Lee-Yoon DS, Liu X, Subjeck J (1996) The 170 kDa glucose regulated stress protein is a large HSP70-, HSP110-like protein of the endoplasmic reticulum. FEBS Lett 380: 68–72 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

