Lessons from lactose permease
- PMID: 16689628
- PMCID: PMC2802108
- DOI: 10.1146/annurev.biophys.35.040405.102005
Lessons from lactose permease
Abstract
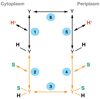
An X-ray structure of the lactose permease of Escherichia coli (LacY) in an inward-facing conformation has been solved. LacY contains N- and C-terminal domains, each with six transmembrane helices, positioned pseudosymmetrically. Ligand is bound at the apex of a hydrophilic cavity in the approximate middle of the molecule. Residues involved in substrate binding and H+ translocation are aligned parallel to the membrane at the same level and may be exposed to a water-filled cavity in both the inward- and outward-facing conformations, thereby allowing both sugar and H+ release directly into either cavity. These structural features may explain why LacY catalyzes galactoside/H+ symport in both directions utilizing the same residues. A working model for the mechanism is presented that involves alternating access of both the sugar- and H+-binding sites to either side of the membrane.
Figures


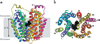

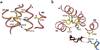


References
-
- Abramson J, Kaback HR, Iwata S. Structural comparison of lactose permease and the glycerol-3-phosphate antiporter: members of the major facilitator superfamily. Curr. Opin. Struct. Biol. 2004;14:413–419. - PubMed
-
- Abramson J, Smirnova I, Kasho V, Verner G, Kaback HR, Iwata S. Structure and mechanism of the lactose permease of Escherichia coli. Science. 2003;301:610–615. - PubMed
-
- Bieseler B, Prinz H, Beyreuther K. Topological studies of lactose permease of Escherichia coli by protein sequence analysis. Ann. N. Y. Acad. Sci. 1985;456:309–325. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
