Nerve growth factor affects Ca2+ currents via the p75 receptor to enhance prolactin mRNA levels in GH3 rat pituitary cells
- PMID: 16690703
- PMCID: PMC1817754
- DOI: 10.1113/jphysiol.2006.110791
Nerve growth factor affects Ca2+ currents via the p75 receptor to enhance prolactin mRNA levels in GH3 rat pituitary cells
Abstract
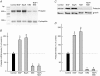
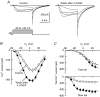
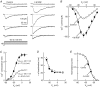
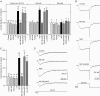
In clonal pituitary GH(3) cells, spontaneous action potentials drive the opening of Ca(v)1 (L-type) channels, leading to Ca(2+) transients that are coupled to prolactin gene transcription. Nerve growth factor (NGF) has been shown to stimulate prolactin synthesis by GH(3) cells, but the underlying mechanisms are unknown. Here we studied whether NGF influences prolactin gene expression and Ca(2+) currents. By using RT-PCR, NGF (50 ng ml(-1)) was found to augment prolactin mRNA levels by approximately 80% when applied to GH(3) cells for 3 days. A parallel change in the prolactin content was detected by Western blotting. Both NGF-induced responses were mimicked by an agonist (Bay K 8644) and prevented by a blocker (nimodipine) of L-type channels. In whole-cell patch-clamp experiments, NGF enhanced the L-type Ca(2+) current by approximately 2-fold within 60 min. This effect reversed quickly upon growth factor withdrawal, but was maintained for days in the continued presence of NGF. In addition, chronic treatment (>or= 24 h) with NGF amplified the T-type current, which flows through Ca(v)3 channels and is thought to support pacemaking activity. Thus, NGF probably increases the amount of Ca(2+) that enters per action potential and may also induce a late increase in spike frequency. MC192, a specific antibody for the p75 neurotrophin receptor, but not tyrosine kinase inhibitors (K252a and lavendustin A), blocked the effects of NGF on Ca(2+) currents. Overall, the results indicate that NGF activates the p75 receptor to cause a prolonged increase in Ca(2+) influx through L-type channels, which in turn up-regulates the prolactin mRNA.
Figures






Similar articles
-
BAY 41-2272, a potent activator of soluble guanylyl cyclase, stimulates calcium elevation and calcium-activated potassium current in pituitary GH cells.Clin Exp Pharmacol Physiol. 2005 Dec;32(12):1078-87. doi: 10.1111/j.1440-1681.2005.04315.x. Clin Exp Pharmacol Physiol. 2005. PMID: 16445574
-
L-type calcium channel activation up-regulates the mRNAs for two different sodium channel alpha subunits (Nav1.2 and Nav1.3) in rat pituitary GH3 cells.Brain Res Mol Brain Res. 2003 Aug 19;116(1-2):115-25. doi: 10.1016/s0169-328x(03)00279-1. Brain Res Mol Brain Res. 2003. PMID: 12941467
-
Multiple acute effects on the membrane potential of PC12 cells produced by nerve growth factor (NGF).J Cell Physiol. 2005 Jun;203(3):501-9. doi: 10.1002/jcp.20309. J Cell Physiol. 2005. PMID: 15729735
-
Intracellular calcium concentration and hormone secretion are controlled differently by TRH in rat neonatal lactotrophs and somatotrophs.J Endocrinol. 1997 Sep;154(3):483-94. doi: 10.1677/joe.0.1540483. J Endocrinol. 1997. PMID: 9379126
-
Ion channels and signaling in the pituitary gland.Endocr Rev. 2010 Dec;31(6):845-915. doi: 10.1210/er.2010-0005. Epub 2010 Jul 21. Endocr Rev. 2010. PMID: 20650859 Free PMC article. Review.
Cited by
-
Regulation of Ca v 3.1 channels by glucocorticoids.Cell Mol Neurobiol. 2009 Dec;29(8):1265-73. doi: 10.1007/s10571-009-9422-2. Cell Mol Neurobiol. 2009. PMID: 19533336 Free PMC article.
-
Chronic atrial ionic remodeling by aldosterone: potentiation of L-type Ca2+ channels and its arrhythmogenic significance.Pflugers Arch. 2016 Nov;468(11-12):1823-1835. doi: 10.1007/s00424-016-1876-8. Epub 2016 Sep 15. Pflugers Arch. 2016. PMID: 27631154
-
Nerve growth factor sensitizes adult sympathetic neurons to the proinflammatory peptide bradykinin.J Neurosci. 2014 Sep 3;34(36):11959-71. doi: 10.1523/JNEUROSCI.1536-14.2014. J Neurosci. 2014. PMID: 25186743 Free PMC article.
-
Upregulation of voltage-gated calcium channel cav1.3 in bovine somatotropes treated with ghrelin.J Signal Transduct. 2013;2013:527253. doi: 10.1155/2013/527253. Epub 2013 Dec 18. J Signal Transduct. 2013. PMID: 24455243 Free PMC article.
-
Nerve growth factor: a neuroimmune crosstalk mediator for all seasons.Immunology. 2017 May;151(1):1-15. doi: 10.1111/imm.12717. Epub 2017 Feb 21. Immunology. 2017. PMID: 28112808 Free PMC article. Review.
References
-
- Aanestad M, Rotnes JS, Torjesen PA, Haug E, Sand O, Bjoro T. Epidermal growth factor stimulates the prolactin synthesis and secretion in rat pituitary cells in culture (GH4C1 cells) by increasing the intracellular concentration of free calcium. Acta Endocrinol. 1993;128:361–366. - PubMed
-
- Arroba AI, Frago LM, Argente J, Chowen JA. Oestrogen requires the insulin-like growth factor-I receptor for stimulation of prolactin synthesis via mitogen-activated protein kinase. J Neuroendocrinol. 2005;17:97–104. - PubMed
-
- Baldelli P, Forni PE, Carbone E. BDNF, NT-3 and NGF induce distinct new Ca2+ channel synthesis in developing hippocampal neurons. Eur J Neurosci. 2000;12:4017–4032. - PubMed
-
- Berg MM, Sternberg DW, Parada LF, Chao MV. K-252a inhibits nerve growth factor-induced trk proto-oncogene tyrosine phosphorylation and kinase activity. J Biol Chem. 1992;267:13–16. - PubMed
-
- Black MJ, Woo Y, Rane SG. Calcium channel upregulation in response to activation of neurotrophin and surrogate neurotrophin receptor tyrosine kinases. J Neurosci Res. 2003;74:23–36. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

