Short-term reorganization of input-deprived motor vibrissae representation following motor disconnection in adult rats
- PMID: 16690708
- PMCID: PMC1817759
- DOI: 10.1113/jphysiol.2006.109116
Short-term reorganization of input-deprived motor vibrissae representation following motor disconnection in adult rats
Abstract
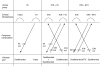
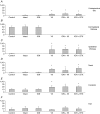
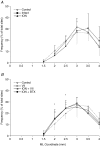
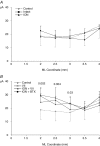
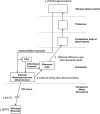
It has been proposed that abnormal vibrissae input to the motor cortex (M1) mediates short-term cortical reorganization after facial nerve lesion. To test this hypothesis, we cut first the infraorbital nerve (ION cut) and then the facial nerve (VII cut) in order to evaluate M1 reorganization without any aberrant, facial-nerve-lesion-induced sensory feedback. In each animal, M1 output was assessed in both hemispheres by mapping movements induced by intracortical microstimulation. M1 output was compared in different types of peripheral manipulations: (i) contralateral intact vibrissal pad (intact hemispheres), (ii) contralateral VII cut (VII hemispheres), (iii) contralateral ION cut (ION hemispheres), (iv) contralateral VII cut after contralateral ION cut (ION + VII hemispheres), (v) contralateral pad botulinum-toxin-injected after ION cut (ION + BTX hemispheres). Right and left hemispheres in untouched animals were the reference for normal M1 map (control hemispheres). Findings demonstrated that: (1) in ION hemispheres, the mean size of the vibrissae representation was not significantly different from those in intact and control hemispheres; (2) reorganization of the vibrissae movement representation clearly emerged only in hemispheres where the contralateral vibrissae pad had undergone motor output disconnection (VII cut hemispheres); (3) the persistent loss of vibrissae input did not change the M1 reorganization pattern during the first 48 h after motor paralysis (ION + VII cut and ION + BTX hemispheres). Thus, after motor paralysis, vibrissa input does not provide the gating signal necessary to trigger M1 reorganization.
Figures





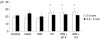
Similar articles
-
Reorganization of vibrissal motor representation following severing and repair of the facial nerve in adult rats.Exp Brain Res. 2000 Mar;131(1):33-43. doi: 10.1007/s002219900297. Exp Brain Res. 2000. PMID: 10759169
-
Persistence of vibrissal motor representation following vibrissal pad deafferentation in adult rats.Exp Brain Res. 2001 Mar;137(2):180-9. doi: 10.1007/s002210000652. Exp Brain Res. 2001. PMID: 11315546
-
Time course of motor cortex reorganization following botulinum toxin injection into the vibrissal pad of the adult rat.Eur J Neurosci. 2002 Oct;16(7):1333-48. doi: 10.1046/j.1460-9568.2002.02195.x. Eur J Neurosci. 2002. PMID: 12405994
-
Anatomical loops and their electrical dynamics in relation to whisking by rat.Somatosens Mot Res. 1999;16(2):69-88. doi: 10.1080/08990229970528. Somatosens Mot Res. 1999. PMID: 10449057 Review.
-
Local cortical interactions determine the form of cortical plasticity.J Neurobiol. 1999 Oct;41(1):58-63. J Neurobiol. 1999. PMID: 10504192 Review.
Cited by
-
Corticospinal output and cortical excitation-inhibition balance in distal hand muscle representations in nonprimary motor area.Hum Brain Mapp. 2011 Oct;32(10):1692-703. doi: 10.1002/hbm.21137. Epub 2010 Sep 30. Hum Brain Mapp. 2011. PMID: 20886574 Free PMC article.
-
Face sensorimotor cortex undergoes neuroplastic changes in a rat model of trigeminal neuropathic pain.Exp Brain Res. 2018 May;236(5):1357-1368. doi: 10.1007/s00221-018-5226-2. Epub 2018 Mar 8. Exp Brain Res. 2018. PMID: 29520443
References
-
- Ahrens KF, Kleinfeld D. Current flow in vibrissa motor cortex can phase-lock with exploratory rhythmic whisking in rat. J Neurophysiol. 2004;92:1700–1707. - PubMed
-
- Angaut P, Cicirata F. Anatomo-functional organization of the neocerebellar control pathways on the cerebral motor cortex. Rev Neurol. 1994;150:39–45. - PubMed
-
- Armstrong-James M, George MJ. Bilateral receptive fields of cells in rat Sm1 cortex. Exp Brain Res. 1988;70:155–165. - PubMed
-
- Asanuma H, Stoney SJ, Abzung C. Relationship between afferent input and motor outflow in cat motorsensory cortex. J Neurophysiol. 1968;31:670–681. - PubMed
-
- Berg WR, Kleinfeld D. Vibrissae movement elicited by rhythmic electrical microstimulation to motor cortex in the aroused rat mimics exploratory whisking. J Neurophysiol. 2003;90:2950–2963. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources

