Hyperpolarization-activated cation channels in fast-spiking interneurons of rat hippocampus
- PMID: 16690716
- PMCID: PMC1817792
- DOI: 10.1113/jphysiol.2005.104042
Hyperpolarization-activated cation channels in fast-spiking interneurons of rat hippocampus
Abstract
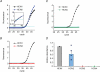
Hyperpolarization-activated channels (Ih or HCN channels) are widely expressed in principal neurons in the central nervous system. However, Ih in inhibitory GABAergic interneurons is less well characterized. We examined the functional properties of Ih in fast-spiking basket cells (BCs) of the dentate gyrus, using hippocampal slices from 17- to 21-day-old rats. Bath application of the Ih channel blocker ZD 7288 at a concentration of 30 microm induced a hyperpolarization of 5.7 +/- 1.5 mV, an increase in input resistance and a correlated increase in apparent membrane time constant. ZD 7288 blocked a hyperpolarization-activated current in a concentration-dependent manner (IC50, 1.4 microm). The effects of ZD 7288 were mimicked by external Cs+. The reversal potential of Ih was -27.4 mV, corresponding to a Na+ to K+ permeability ratio (PNa/PK) of 0.36. The midpoint potential of the activation curve of Ih was -83.9 mV, and the activation time constant at -120 mV was 190 ms. Single-cell expression analysis using reverse transcription followed by quantitative polymerase chain reaction revealed that BCs coexpress HCN1 and HCN2 subunit mRNA, suggesting the formation of heteromeric HCN1/2 channels. ZD 7288 increased the current threshold for evoking antidromic action potentials by extracellular stimulation, consistent with the expression of Ih in BC axons. Finally, ZD 7288 decreased the frequency of miniature inhibitory postsynaptic currents (mIPSCs) in hippocampal granule cells, the main target cells of BCs, to 70 +/- 4% of the control value. In contrast, the amplitude of mIPSCs was unchanged, consistent with the presence of Ih in inhibitory terminals. In conclusion, our results suggest that Ih channels are expressed in the somatodendritic region, axon and presynaptic elements of fast-spiking BCs in the hippocampus.
Figures



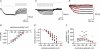

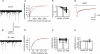
Comment in
-
Queer channels in hippocampal basket cells: h-current without sag.J Physiol. 2006 Jul 1;574(Pt 1):2. doi: 10.1113/jphysiol.2006.113407. Epub 2006 May 18. J Physiol. 2006. PMID: 16709628 Free PMC article. No abstract available.
References
-
- Awatramani GB, Price GD, Trussell LO. Modulation of transmitter release by presynaptic resting potential and background calcium levels. Neuron. 2005;48:109–121. - PubMed
-
- Beaumont V, Zucker RS. Enhancement of synaptic transmission by cyclic AMP modulation of presynaptic Ih channels. Nat Neurosci. 2000;3:133–141. - PubMed
-
- Buhl EH, Halasy K, Somogyi P. Diverse sources of hippocampal unitary inhibitory postsynaptic potentials and the number of synaptic release sites. Nature. 1994;368:823–828. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

