Oogenesis requires germ cell-specific transcriptional regulators Sohlh1 and Lhx8
- PMID: 16690745
- PMCID: PMC1472434
- DOI: 10.1073/pnas.0601083103
Oogenesis requires germ cell-specific transcriptional regulators Sohlh1 and Lhx8
Abstract
Mammalian oogenesis requires oocyte-specific transcriptional regulators. The full complement of oocyte-specific transcription factors is unknown. Here, we describe the finding that Sohlh1, a spermatogenesis and oogenesis basic helix-loop-helix transcription factor in females, is preferentially expressed in oocytes and required for oogenesis. Sohlh1 disruption perturbs follicular formation in part by causing down-regulation of two genes that are known to disrupt folliculogenesis: newborn ovary homeobox gene (Nobox) and factor in the germ-line alpha (Figla). In addition, we show that Lhx8 is downstream of Sohlh1 and critical in fertility. Thus, Sohlh1 and Lhx8 are two germ cell-specific, critical regulators of oogenesis.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures
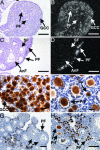
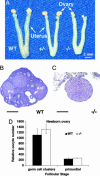



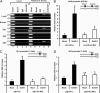
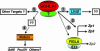
References
-
- Pepling M. E., Spradling A. C. Dev. Biol. 2001;234:339–351. - PubMed
-
- Wylie C. Cell. 1999;96:165–174. - PubMed
-
- Rajkovic A., Pangas S. A., Ballow D., Suzumori N., Matzuk M. M. Science. 2004;305:1157–1159. - PubMed
-
- Dong J., Albertini D. F., Nishimori K., Kumar T. R., Lu N., Matzuk M. M. Nature. 1996;383:531–535. - PubMed
-
- Dean J. J. Reprod. Immunol. 2002;53:171–180. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

