Steroid and xenobiotic receptor and vitamin D receptor crosstalk mediates CYP24 expression and drug-induced osteomalacia
- PMID: 16691293
- PMCID: PMC1459072
- DOI: 10.1172/JCI27793
Steroid and xenobiotic receptor and vitamin D receptor crosstalk mediates CYP24 expression and drug-induced osteomalacia
Abstract
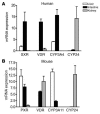
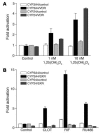
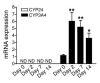
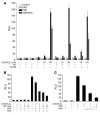
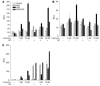
The balance between bioactivation and degradation of 1,25-dihydroxyvitamin D3 [1,25(OH)2D3] is critical for ensuring appropriate biological effects of vitamin D. Cytochrome P450, family 24-mediated (CYP24-mediated) 24-hydroxylation of 1,25(OH)2D3 is an important step in the catabolism of 1,25(OH)2D3. The enzyme is directly regulated by vitamin D receptor (VDR), and it is expressed mainly in the kidney, where VDR is also abundant. A recent report suggests that activation of steroid and xenobiotic receptor (SXR) also enhances the expression of CYP24, providing a new molecular mechanism of drug-induced osteomalacia. However, here we showed that activation of SXR did not induce CYP24 expression in vitro and in vivo, nor did it transactivate the CYP24 promoter. Instead, SXR inhibited VDR-mediated CYP24 promoter activity, and CYP24 expression was very low in tissues containing high levels of SXR, including the small intestine. Moreover, 1,25(OH)2D3-induced CYP24 expression was enhanced in mice lacking the SXR ortholog pregnane X receptor, and treatment of humans with the SXR agonist rifampicin had no effect on intestinal CYP24 expression, despite demonstration of marked CYP3A4 induction. Combined with our previous findings that CYP3A4, not CYP24, plays the dominant role in hydroxylation of 1,25(OH)2D3 in human liver and intestine, our results indicate that SXR has a dual role in mediating vitamin D catabolism and drug-induced osteomalacia.
Figures

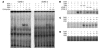

Comment in
-
Response to Zhou et al. Osteomalacia is a frequent complication resulting from long-term therapy with drugs such as phenytoin, carbamazepine, and phenobarbital.J Clin Invest. 2006 Oct;116(10):2564. doi: 10.1172/JCI30017. J Clin Invest. 2006. PMID: 17016548 Free PMC article. No abstract available.
References
-
- Pike J.W., Shevde N.K. Vitamin D. 2nd edition. D. Feldman, F.H. Glorieux, and J.W. Pike, editors. Elsevier Academic Press.; Boston, Massachusetts, USA.: 2005. The vitamin D receptor. pp. 167–191.
-
- Jones G., Strugnell S.A., DeLuca H.F. Current understanding of the molecular actions of vitamin D. Physiol. Rev. 1998;78:1193–1231. - PubMed
-
- Sutton A.L., MacDonald P.N. Vitamin D: more than a “bone-a-fide” hormone. Mol. Endocrinol. 2003;17:777–791. - PubMed
-
- Nagpal S., Na S., Rathnachalam R. Noncalcemic actions of vitamin D receptor ligands. Endocr. Rev. 2005;26:662–687. - PubMed
-
- Dusso A.S., Brown A.J., Slatopolsky E. Vitamin D. Am. J. Physiol. Renal Physiol. 2005;289:F8–F28. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 GM060346/GM/NIGMS NIH HHS/United States
- R37 GM038149/GM/NIGMS NIH HHS/United States
- M01 RR000046/RR/NCRR NIH HHS/United States
- P01 GM032165/GM/NIGMS NIH HHS/United States
- GM32165/GM/NIGMS NIH HHS/United States
- GM60572/GM/NIGMS NIH HHS/United States
- N01-DK-9-2310/DK/NIDDK NIH HHS/United States
- R01 GM063666/GM/NIGMS NIH HHS/United States
- RR000046/RR/NCRR NIH HHS/United States
- GM60346/GM/NIGMS NIH HHS/United States
- ES07033/ES/NIEHS NIH HHS/United States
- GM38149/GM/NIGMS NIH HHS/United States
- R01 GM060572/GM/NIGMS NIH HHS/United States
- P30 ES007033/ES/NIEHS NIH HHS/United States
- GM63666/GM/NIGMS NIH HHS/United States

