Cross-linking of the human DNA repair protein O6-alkylguanine DNA alkyltransferase to DNA in the presence of 1,2,3,4-diepoxybutane
- PMID: 16696566
- PMCID: PMC3213031
- DOI: 10.1021/tx0600088
Cross-linking of the human DNA repair protein O6-alkylguanine DNA alkyltransferase to DNA in the presence of 1,2,3,4-diepoxybutane
Abstract
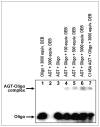
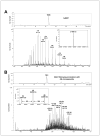
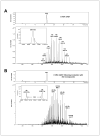
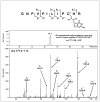
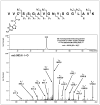
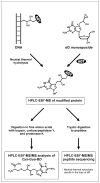
1,2,3,4-Diepoxybutane (DEB) is a key carcinogenic metabolite of the important industrial chemical 1,3-butadiene. DEB is a bifunctional alkylating agent capable of reacting with DNA and proteins. Initial DNA alkylation by DEB produces N7-(2'-hydroxy-3',4'-epoxybut-1'-yl)-guanine monoadducts, which can react with another nucleophilic site to form cross-linked adducts. A recent report revealed a strong correlation between cellular expression of the DNA repair protein O6-alkylguanine DNA alkyltransferase (AGT) and the cytotoxic and mutagenic activity of DEB, suggesting that DEB induces AGT-DNA cross-links (Valadez, J. G., et al. (2004) Activation of bis-electrophiles to mutagenic conjugates by human O6-alkylguanine-DNA alkyltransferase. Chem. Res. Toxicol. 17, 972-982). The purpose of our study was to analyze the formation and structures of DEB-induced AGT-DNA conjugates and to identify specific amino acid residues within the protein involved in cross-linking. DNA-protein cross-link formation was detected by SDS-PAGE when 32P-labeled double-stranded oligodeoxynucleotides were exposed to DEB in the presence of either wild-type hAGT or a C145A hAGT mutant. Capillary HPLC-electrospray ionization mass spectrometry (ESI-MS) analysis of hAGT that had been treated with N7-(2'-hydroxy-3',4'-epoxybut-1'-yl)-deoxyguanosine (dG monoepoxide) revealed the ability of the protein to form either one or two butanediol-dG cross-links, corresponding to mass shifts of +353 and +706 Da, respectively. HPLC-ESI+ -MS/MS sequencing of the tryptic peptides obtained from dG monoepoxide-treated protein indicated that the two cross-linking sites were the alkyl acceptor site, Cys145, and a neighboring active site residue, Cys150. The same two amino acid residues of hAGT became covalently cross-linked to DNA following DEB treatment. Modification of Cys145 was further confirmed by HPLC-ESI+ -MS/MS analysis of dG monoepoxide-treated synthetic peptide GNPVPILIPCHR which represents the active site tryptic fragment of hAGT (C = Cys145). The replacement of the catalytic cysteine residue with alanine in the C145A hAGT mutant abolished DEB-induced cross-linking at this site, while the formation of conjugates via neighboring Cys150 was retained. The exact chemical structure of the cross-linked lesion was established as 1-(S-cysteinyl)-4-(guan-7-yl)-2,3-butanediol by HPLC-ESI+ -MS/MS analysis of the amino acids resulting from the total digestion of modified proteins analyzed in parallel with an authentic standard. AGT-DNA cross-linking is a likely mechanism of DEB-mediated cytotoxicity in cells expressing this important repair protein.
Figures






References
-
- Pegg AE. Repair of O6-alkylguanine by alkyltransferases. Mutat Res. 2000;462:83–100. - PubMed
-
- Daniels DS, Woo TT, Luu KX, Noll DM, Clarke ND, Pegg AE, Tainer JA. DNA binding and nucleotide flipping by the human DNA repair protein AGT. Nat Struct Mol Biol. 2004;11:714–720. - PubMed
-
- Federwisch M, Hassiepen U, Bender K, Dewor M, Rajewsky MF, Wollmer A. Recombinant human O6-alkylguanine-DNA alkyltransferase (AGT), Cys145-alkylated AGT and Cys145 --> Met145 mutant AGT: comparison by isoelectric focusing, CD and time-resolved fluorescence spectroscopy. Biochem J. 1997;324 (Pt 1):321–328. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

