Polycyclic aromatic hydrocarbon (PAH) o-quinones produced by the aldo-keto-reductases (AKRs) generate abasic sites, oxidized pyrimidines, and 8-oxo-dGuo via reactive oxygen species
- PMID: 16696575
- PMCID: PMC2366214
- DOI: 10.1021/tx0600245
Polycyclic aromatic hydrocarbon (PAH) o-quinones produced by the aldo-keto-reductases (AKRs) generate abasic sites, oxidized pyrimidines, and 8-oxo-dGuo via reactive oxygen species
Abstract
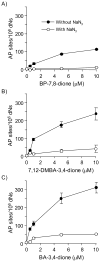
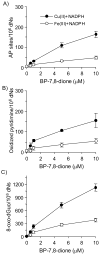
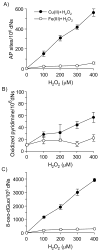
Reactive and redox-active polycyclic aromatic hydrocarbon (PAH) o-quinones produced by Aldo-Keto Reductases (AKRs) have the potential to cause depurinating adducts leading to the formation of abasic sites and oxidative base lesions. The aldehyde reactive probe (ARP) was used to detect these lesions in calf thymus DNA treated with three PAH o-quinones (BP-7,8-dione, 7,12-DMBA-3,4-dione, and BA-3,4-dione) in the absence and presence of redox-cycling conditions. In the absence of redox-cycling, a modest amount of abasic sites were detected indicating the formation of a low level of covalent o-quinone depurinating adducts (>3.2 x 10(6) dNs). In the presence of NADPH and CuCl2, the three PAH o-quinones increased the formation of abasic sites due to ROS-derived lesions destabilizing the N-glycosidic bond. The predominant source of AP sites, however, was revealed by coupling the assay with human 8-oxoguanine glycosylase (hOGG1) treatment, showing that 8-oxo-dGuo was the major lesion caused by PAH o-quinones. The levels of 8-oxo-dGuo formation were independently validated by HPLC-ECD analysis. Apyrimidinic sites were also revealed by coupling the assay with Escherichia coli (Endo III) treatment showing that oxidized pyrimidines were formed, but to a lesser extent. Different mechanisms were responsible for the formation of the oxidative lesions depending on whether Cu(II) or Fe(III) was used in the redox-cycling conditions. In the presence of Cu(II)-mediated PAH o-quinone redox-cycling, catalase completely suppressed the formation of the lesions, but mannitol and sodium benzoate were without effect. By contrast, sodium azide, which acts as a *OH and 1O2 scavenger, inhibited the formation of all oxidative lesions, suggesting that the ROS responsible was 1O2. However, in the presence of Fe(III)-mediated PAH o-quinone redox-cycling, the *OH radical scavengers and sodium azide consistently attenuated their formation, indicating that the ROS responsible was *OH.
Figures






References
-
- Xue W, Warshawsky D. Metabolic activation of polycyclic and heterocyclic aromatic hydrocarbons and DNA damage. Toxicol Appl Pharmacol. 2005;206:73–93. - PubMed
-
- Rothman N, Poirier MC, Baser ME, Hansen JA, Gentile C, Bowman ED, Strickland PT. Formation of polycyclic aromatic hydrocarbon-DNA adducts in peripheral white blood cells during consumption of charcoal-broiled beef. Carcinogenesis. 1990;11:1241–1243. - PubMed
-
- Denissenko MF, Pao A, Tang M, Pfeifer GP. Preferential formation of benzo[a]pyrene adducts at lung cancer mutational hotspots in P53. Science. 1996;274:430–432. - PubMed
-
- Gelboin HV. Benzo[a]pyrene metabolism, activation and carcinogenesis: role and regulation of mixed function oxidases and related enzymes. Physiol Rev. 1980;60:1107–1166. - PubMed
-
- Conney AH. Induction of microsomal enzymes by foreign chemicals and carcinogenesis by polycyclic aromatic hydrocarbons. G H A Clowes Memorial Lecture. Cancer Res. 1982;42:4875–4917. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

