Molecular morphology and toxicity of cytoplasmic prion protein aggregates in neuronal and non-neuronal cells
- PMID: 16696854
- PMCID: PMC2954960
- DOI: 10.1111/j.1471-4159.2006.03837.x
Molecular morphology and toxicity of cytoplasmic prion protein aggregates in neuronal and non-neuronal cells
Abstract
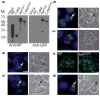
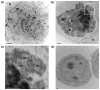
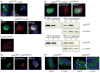
Recent studies have revealed that accumulation of prion protein (PrP) in the cytoplasm results in the production of aggregates that are insoluble in non-ionic detergents and partially resistant to proteinase K. Transgenic mice expressing PrP in the cytoplasm develop severe ataxia with cerebellar degeneration and gliosis, suggesting that cytoplasmic PrP may play a role in the pathogenesis of prion diseases. The mechanism of cytoplasmic PrP neurotoxicity is not known. In this report, we determined the molecular morphology of cytoplasmic PrP aggregates by immunofluorescence and electron microscopy, in neuronal and non-neuronal cells. Transient expression of cytoplasmic PrP produced juxtanuclear aggregates reminiscent of aggresomes in human embryonic kidney 293 cells, human neuroblastoma BE2-M17 cells and mouse neuroblastoma N2a cells. Time course studies revealed that discrete aggregates form first throughout the cytoplasm, and then coalesce to form an aggresome. Aggresomes containing cytoplasmic PrP were 1-5-microm inclusion bodies and were filled with electron-dense particles. Cytoplasmic PrP aggregates induced mitochondrial clustering, reorganization of intermediate filaments, prevented the secretion of wild-type PrP molecules and diverted these molecules to the cytoplasm. Cytoplasmic PrP decreased the viability of neuronal and non-neuronal cells. We conclude that any event leading to accumulation of PrP in the cytoplasm is likely to result in cell death.
Figures



References
-
- Bounhar Y, Zhang Y, Goodyer CG, LeBlanc A. Prion protein protects human neurons against Bax-mediated apoptosis. J Biol Chem. 2001;276:39145–39149. - PubMed
-
- Brandner S, Isenmann S, Raeber A, Fischer M, Sailer A, Kobayashi Y, Marino S, Weissmann C, Aguzzi A. Normal host prion protein necessary for scrapie-induced neurotoxicity. Nature. 1996;379:339–343. - PubMed
-
- Debure L, Vayssiere JL, Rincheval V, Loison F, Le Drean Y, Michel D. Intracellular clusterin causes juxtanuclear aggregate formation and mitochondrial alteration. J Cell Sci. 2003;116:3109–3121. - PubMed
-
- Drisaldi B, Stewart RS, Adles C, Stewart LR, Quaglio E, Biasini E, Fioriti L, Chiesa R, Harris DA. Mutant PrP is delayed in its exit from the endoplasmic reticulum, but neither wild-type nor mutant PrP undergoes retrotranslocation prior to proteasomal degradation. J Biol Chem. 2003;278:21732–21743. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

