Fourier transform infrared spectroscopy provides a fingerprint for the tetramer and for the aggregates of transthyretin
- PMID: 16698785
- PMCID: PMC1563765
- DOI: 10.1529/biophysj.106.085928
Fourier transform infrared spectroscopy provides a fingerprint for the tetramer and for the aggregates of transthyretin
Abstract
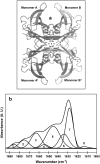
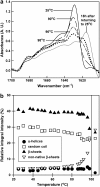
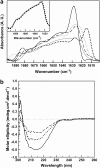
Transthyretin (TTR) is an amyloidogenic protein whose aggregation is responsible for several familial amyloid diseases. Here, we use FTIR to describe the secondary structural changes that take place when wt TTR undergoes heat- or high-pressure-induced denaturation, as well as fibril formation. Upon thermal denaturation, TTR loses part of its intramolecular beta-sheet structure followed by an increase in nonnative, probably antiparallel beta-sheet contacts (bands at 1,616 and 1,686 cm(-1)) and in the light scattering, suggesting its aggregation. Pressure-induced denaturation studies show that even at very elevated pressures (12 kbar), TTR loses only part of its beta-sheet structure, suggesting that pressure leads to a partially unfolded species. On comparing the FTIR spectrum of the TTR amyloid fibril produced at atmospheric pressure upon acidification (pH 4.4) with the one presented by the native tetramer, we find that the content of beta-sheets does not change much upon fibrillization; however, the alignment of beta-sheets is altered, resulting in the formation of distinct beta-sheet contacts (band at 1,625 cm(-1)). The random-coil content also decreases in going from tetramers to fibrils. This means that, although part of the tertiary- and secondary-structure content of the TTR monomers has to be lost before fibril formation, as previously suggested, there must be a subsequent reorganization of part of the random-coil structure into a well-organized structure compatible with the amyloid fibril, as well as a readjustment of the alignment of the beta-sheets. Interestingly, the infrared spectrum of the protein recovered from a cycle of compression-decompression at pD 5, 37 degrees C, is quite similar to that of fibrils produced at atmospheric pressure (pH 4.4), which suggests that high hydrostatic pressure converts the tetramers of TTR into an amyloidogenic conformation.
Figures


Similar articles
-
The acid-mediated denaturation pathway of transthyretin yields a conformational intermediate that can self-assemble into amyloid.Biochemistry. 1996 May 21;35(20):6470-82. doi: 10.1021/bi952501g. Biochemistry. 1996. PMID: 8639594
-
Characterization of the transthyretin acid denaturation pathways by analytical ultracentrifugation: implications for wild-type, V30M, and L55P amyloid fibril formation.Biochemistry. 1998 Dec 22;37(51):17851-64. doi: 10.1021/bi981876+. Biochemistry. 1998. PMID: 9922152
-
Partial denaturation of transthyretin is sufficient for amyloid fibril formation in vitro.Biochemistry. 1992 Sep 15;31(36):8654-60. doi: 10.1021/bi00151a036. Biochemistry. 1992. PMID: 1390650
-
Transthyretin quaternary and tertiary structural changes facilitate misassembly into amyloid.Adv Protein Chem. 1997;50:161-81. doi: 10.1016/s0065-3233(08)60321-6. Adv Protein Chem. 1997. PMID: 9338081 Review.
-
ATR-FTIR: a "rejuvenated" tool to investigate amyloid proteins.Biochim Biophys Acta. 2013 Oct;1828(10):2328-38. doi: 10.1016/j.bbamem.2013.04.012. Epub 2013 Jun 5. Biochim Biophys Acta. 2013. PMID: 23746423 Review.
Cited by
-
Inhibition of human transthyretin aggregation by non-steroidal anti-inflammatory compounds: a structural and thermodynamic analysis.Int J Mol Sci. 2013 Mar 6;14(3):5284-311. doi: 10.3390/ijms14035284. Int J Mol Sci. 2013. PMID: 23466880 Free PMC article.
-
Molecular Spectroscopic Markers of Abnormal Protein Aggregation.Molecules. 2020 May 27;25(11):2498. doi: 10.3390/molecules25112498. Molecules. 2020. PMID: 32471300 Free PMC article. Review.
-
Stable misfolded states of human serum albumin revealed by high-pressure infrared spectroscopic studies.Eur Biophys J. 2008 Sep;37(7):1127-32. doi: 10.1007/s00249-008-0277-0. Epub 2008 Feb 15. Eur Biophys J. 2008. PMID: 18274741
-
The impact of solubility and electrostatics on fibril formation by the H3 and H4 histones.Protein Sci. 2011 Dec;20(12):2060-73. doi: 10.1002/pro.743. Epub 2011 Nov 9. Protein Sci. 2011. PMID: 21953551 Free PMC article.
-
The effect of Aβ on IAPP aggregation in the presence of an isolated β-cell membrane.J Mol Biol. 2012 Aug 10;421(2-3):348-63. doi: 10.1016/j.jmb.2012.01.048. Epub 2012 Feb 1. J Mol Biol. 2012. PMID: 22321797 Free PMC article.
References
-
- Blake, C. C., M. J. Geisow, S. J. Oatley, B. Rerat, and C. Rerat. 1978. Structure of prealbumin: secondary, tertiary and quaternary interactions determined by Fourier refinement at 1.8 Å. J. Mol. Biol. 121:339–356. - PubMed
-
- Sousa, M. M., and M. J. Saraiva. 2003. Neurodegeneration in familial amyloid polyneuropathy: from pathology to molecular signaling. Prog. Neurobiol. 71:385–400. - PubMed
-
- Robbins, J., and J. E. Rall. 1960. Proteins associated with the thyroid hormones. Physiol. Rev. 40:415–489. - PubMed
-
- van Jaarsveld, P., W. T. Branch, J. Robbins, F. J. Morgan, Y. Kanda, and R. E. Canfield. 1973. Polymorphism of Rhesus monkey serum prealbumin. Purification and partial structure. J. Biol. Chem. 248:7898–7903. - PubMed
-
- Almeida, M. R., and M. J. Saraiva. 1996. Thyroxine binding to transthyretin (TTR) variants-two variants (TTR Pro 55 and TTR Met 111) with a particularly low binding affinity. Eur. J. Endocrinol. 2:226–230. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

