Surface position, not signaling from surrounding maternal tissues, specifies aleurone epidermal cell fate in maize
- PMID: 16698897
- PMCID: PMC1489889
- DOI: 10.1104/pp.106.080945
Surface position, not signaling from surrounding maternal tissues, specifies aleurone epidermal cell fate in maize
Erratum in
- Plant Physiol. 2006 Dec;142(4):1771
Abstract
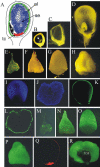
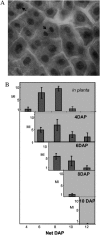
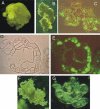
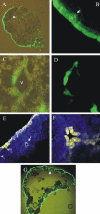
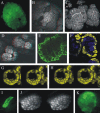
Maize (Zea mays) endosperm consists of an epidermal-like surface layer of aleurone cells, an underlying body of starchy endosperm cells, and a basal layer of transfer cells. To determine whether surrounding maternal tissues perform a role in specifying endosperm cell fates, a maize endosperm organ culture technique was established whereby the developing endosperm is completely removed from surrounding maternal tissues. Using cell type-specific fluorescence markers, we show that aleurone cell fate specification occurs exclusively in response to surface position and does not require specific, continued maternal signal input. The starchy endosperm and aleurone cell fates are freely interchangeable throughout the lifespan of the endosperm, with internalized aleurone cells converting to starchy endosperm cells and with starchy endosperm cells that become positioned at the surface converting to aleurone cells. In contrast to aleurone and starchy endosperm cells, transfer cells fail to develop in in vitro-grown endosperm, supporting earlier indications that maternal tissue interaction is required to fully differentiate this cell type. Several parameters confirm that the maize endosperm organ cultures described herein retain the main developmental features of in planta endosperm, including fidelity of aleurone mutant phenotypes, temporal and spatial control of cell type-specific fluorescent markers, specificity of cell type transcripts, and control of mitotic cell divisions.
Figures

References
-
- Ahn J-W, Kim M, Lim JH, Kim G-T, Pai H-S (2004) Phytocalpain controls the proliferation and differentiation fates of cells in plant organ development. Plant J 38: 969–981 - PubMed
-
- Becraft PW, Asuncion-Crabb Y (2000) Positional cues specify and maintain aleurone cell fate in maize endosperm development. Development 127: 4039–4048 - PubMed
-
- Becraft PW, Stinard PS, McCarty D (1996) CRINKLY4: a TNFR-like receptor kinase involved in maize epidermal differentiation. Science 273: 1406–1409 - PubMed
-
- Cao X, Li K, Suh SG, Guo T, Becraft PW (2005) Molecular analysis of the CRINKLY4 gene family in Arabidopsis thaliana. Planta 220: 645–657 - PubMed
-
- Castro MS, Fontes W, Morhy L, Bloch CJ (1996) Complete amino acid sequences of two gamma-thionins from maize (Zea mays L.) seeds. Protein Pept Lett 3: 267–274
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources

