Low-dose alcohol actions on alpha4beta3delta GABAA receptors are reversed by the behavioral alcohol antagonist Ro15-4513
- PMID: 16698930
- PMCID: PMC1482527
- DOI: 10.1073/pnas.0600194103
Low-dose alcohol actions on alpha4beta3delta GABAA receptors are reversed by the behavioral alcohol antagonist Ro15-4513
Abstract
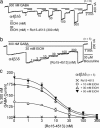
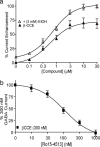
Although it is now more than two decades since it was first reported that the imidazobenzodiazepine Ro15-4513 reverses behavioral alcohol effects, the molecular target(s) of Ro15-4513 and the mechanism of alcohol antagonism remain elusive. Here, we show that Ro15-4513 blocks the alcohol enhancement on recombinant "extrasynaptic" alpha4/6beta3delta GABA(A) receptors at doses that do not reduce the GABA-induced Cl(-) current. At low ethanol concentrations (< or =30 mM), the Ro15-4513 antagonism is complete. However, at higher ethanol concentrations (> or =100 mM), there is a Ro15-4513-insensitive ethanol enhancement that is abolished in receptors containing a point mutation in the second transmembrane region of the beta3 subunit (beta3N265M). Therefore, alpha4/6beta3delta GABA receptors have two distinct alcohol modulation sites: (i) a low-dose ethanol site present in alpha4/6beta3delta receptors that is antagonized by the behavioral alcohol antagonist Ro15-4513 and (ii) a site activated at high (anesthetic) alcohol doses, defined by mutations in membrane-spanning regions. Receptors composed of alpha4beta3N265Mdelta subunits that lack the high-dose alcohol site show a saturable ethanol dose-response curve with a half-maximal enhancement at 16 mM, close to the legal blood alcohol driving limit in most U.S. states (17.4 mM). Like in behavioral experiments, the alcohol antagonist effect of Ro15-4513 on recombinant alpha4beta3delta receptors is blocked by flumazenil and beta-carboline-ethyl ester (beta-CCE). Our findings suggest that ethanol/Ro15-4513-sensitive GABA(A) receptors are important mediators of behavioral alcohol effects.
Conflict of interest statement
Conflict of interest statement: M.W., R.W.O. and H.J.H. have filed a U.S. Provisional Patent Application, Serial No. 60/693,844.
Figures

Comment in
-
Alcohol-sensitive GABA receptors and alcohol antagonists.Proc Natl Acad Sci U S A. 2006 May 30;103(22):8307-8. doi: 10.1073/pnas.0602862103. Epub 2006 May 22. Proc Natl Acad Sci U S A. 2006. PMID: 16717187 Free PMC article. No abstract available.
References
-
- Popp R. L., Lickteig R. L., Lovinger D. M. J. Pharmacol. Exp. Ther. 1999;289:1564–1574. - PubMed
-
- Davies D. L., Trudell J. R., Mihic S. J., Crawford D. K., Alkana R. L. Alcohol Clin. Exp. Res. 2003;27:743–755. - PubMed
-
- Ikeda K., Kobayashi T., Kumanishi T., Yano R., Sora I., Niki H. Neurosci. Res. 2002;44:121–131. - PubMed
-
- Liljequist S., Engel J. Psychopharmacology. 1982;78:71–75. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

