PKCepsilon increases endothelin converting enzyme activity and reduces amyloid plaque pathology in transgenic mice
- PMID: 16698938
- PMCID: PMC1472455
- DOI: 10.1073/pnas.0509725103
PKCepsilon increases endothelin converting enzyme activity and reduces amyloid plaque pathology in transgenic mice
Abstract
Deposition of plaques containing amyloid beta (Abeta) peptides is a neuropathological hallmark of Alzheimer's disease (AD). Here we demonstrate that neuronal overexpression of the epsilon isozyme of PKC decreases Abeta levels, plaque burden, and plaque-associated neuritic dystrophy and reactive astrocytosis in transgenic mice expressing familial AD-mutant forms of the human amyloid precursor protein (APP). Compared with APP singly transgenic mice, APP/PKCepsilon doubly transgenic mice had decreased Abeta levels but showed no evidence for altered cleavage of APP. Instead, PKCepsilon overexpression selectively increased the activity of endothelin-converting enzyme, which degrades Abeta. The activities of other Abeta-degrading enzymes, insulin degrading enzyme and neprilysin, were unchanged. These results indicate that increased neuronal PKCepsilon activity can promote Abeta clearance and reduce AD neuropathology through increased endothelin-converting enzyme activity.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures
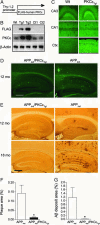
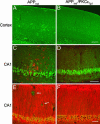
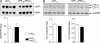

Comment in
-
Another weapon against amyloid.Proc Natl Acad Sci U S A. 2006 May 23;103(21):7943-4. doi: 10.1073/pnas.0602860103. Epub 2006 May 16. Proc Natl Acad Sci U S A. 2006. PMID: 16705031 Free PMC article. No abstract available.
References
-
- Selkoe D. J. Physiol. Rev. 2001;81:741–766. - PubMed
-
- Xu H., Greengard P., Gandy S. J. Biol. Chem. 1995;270:23243–23245. - PubMed
-
- Skovronsky D. M., Moore D. B., Milla M. E., Doms R. W., Lee V. M. J. Biol. Chem. 2000;275:2568–2575. - PubMed
-
- Selkoe D. J., Schenk D. Annu. Rev. Pharmacol. Toxicol. 2003;43:545–584. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

