Synaptic tetraspan vesicle membrane proteins are conserved but not needed for synaptogenesis and neuronal function in Caenorhabditis elegans
- PMID: 16698939
- PMCID: PMC1570102
- DOI: 10.1073/pnas.0509400103
Synaptic tetraspan vesicle membrane proteins are conserved but not needed for synaptogenesis and neuronal function in Caenorhabditis elegans
Abstract
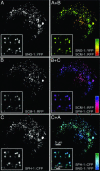
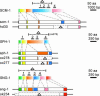
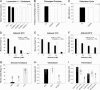
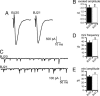
Tetraspan vesicle membrane proteins (TVPs) comprise a major portion of synaptic vesicle proteins, yet their contribution to the synaptic vesicle cycle is poorly understood. TVPs are grouped in three mammalian gene families: physins, gyrins, and secretory carrier-associated membrane proteins (SCAMPs). In Caenorhabditis elegans, only a single member of each of these families exists. These three nematode TVPs colocalize to the same vesicular compartment when expressed in mammalian cells, suggesting that they could serve overlapping functions. To examine their function, C. elegans null mutants were isolated for each gene, and a triple mutant was generated. Surprisingly, these animals develop normally and exhibit normal neuronal architecture and synaptic contacts. In addition, functions of the motor and sensory systems are normal as determined by pharmacological, chemotaxis, and thermotaxis assays. Finally, direct electrophysiological analysis of the neuromuscular junction revealed no phenotype in the TVP mutants. We therefore conclude that TVPs are not needed for the basic neuronal machinery and instead may contribute to subtle higher order functions.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures


References
-
- Südhof T. C. Annu. Rev. Neurosci. 2004;27:509–547. - PubMed
-
- Hübner K., Windoffer R., Hutter H., Leube R. E. Int. Rev. Cytol. 2002;214:103–159. - PubMed
-
- Eshkind L. G., Leube R. E. Cell Tissue Res. 1995;282:423–433. - PubMed
-
- Fernandez-Chacon R., Alvarez de Toledo G., Hammer R. E., Südhof T. C. J. Biol. Chem. 1999;274:32551–32554. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

